Spinal Vascular Shunts: A Patterned Approach
- PMID: 34649916
- PMCID: PMC8805746
- DOI: 10.3174/ajnr.A7312
Spinal Vascular Shunts: A Patterned Approach
Abstract
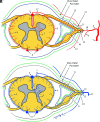
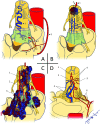
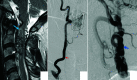
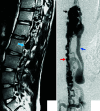
Spinal vascular shunts, including fistulas and malformations, are rare and complex vascular lesions for which multiple classification schemes have been proposed. The most widely adopted scheme consists of 4 types: type I, dural AVFs; type II, intramedullary glomus AVMs; type III, juvenile/metameric AVMs; and type IV, intradural perimedullary AVFs. MR imaging and angiography techniques permit detailed assessment of spinal arteriovenous shunts, though DSA is the criterion standard for delineating vascular anatomy and treatment planning. Diagnosis is almost exclusively based on imaging, and features often mimic more common pathologies. The radiologist's recognition of spinal vascular shunts may improve outcomes because patients may benefit from early intervention.
© 2021 by American Journal of Neuroradiology.
Figures



References
-
- Di Chiro GJ, Doppman L, Ommaya AK. Radiology of spinal cord arteriovenous malformations. In: Di Chiro GJ, Doppman L, Ommaya AK. Progress in Neurological Surgery. Karger Publishers; 1971;4:329–54 10.1159/000391825 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
