Matrigel 3D bioprinting of contractile human skeletal muscle models recapitulating exercise and pharmacological responses
- PMID: 34650188
- PMCID: PMC8516940
- DOI: 10.1038/s42003-021-02691-0
Matrigel 3D bioprinting of contractile human skeletal muscle models recapitulating exercise and pharmacological responses
Abstract
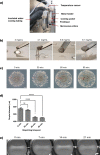
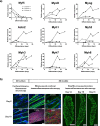
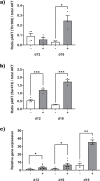
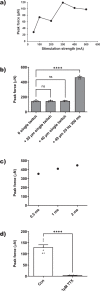
A key to enhance the low translatability of preclinical drug discovery are in vitro human three-dimensional (3D) microphysiological systems (MPS). Here, we show a new method for automated engineering of 3D human skeletal muscle models in microplates and functional compound screening to address the lack of muscle wasting disease medication. To this end, we adapted our recently described 24-well plate 3D bioprinting platform with a printhead cooling system to allow microvalve-based drop-on-demand printing of cell-laden Matrigel containing primary human muscle precursor cells. Mini skeletal muscle models develop within a week exhibiting contractile, striated myofibers aligned between two attachment posts. As an in vitro exercise model, repeated high impact stimulation of contractions for 3 h by a custom-made electrical pulse stimulation (EPS) system for 24-well plates induced interleukin-6 myokine expression and Akt hypertrophy pathway activation. Furthermore, the known muscle stimulators caffeine and Tirasemtiv acutely increase EPS-induced contractile force of the models. This validated new human muscle MPS will benefit development of drugs against muscle wasting diseases. Moreover, our Matrigel 3D bioprinting platform will allow engineering of non-self-organizing complex human 3D MPS.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Rooks, D. et al. Treatment of sarcopenia with bimagrumab: results from a phase II, randomized, controlled, proof-of-concept study. J. Am. Geriatr. Soc., 10.1111/jgs.14927 (2017). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

