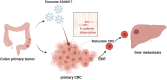
Exosome-Derived ADAM17 Promotes Liver Metastasis in Colorectal Cancer
- PMID: 34650435
- PMCID: PMC8506248
- DOI: 10.3389/fphar.2021.734351
Exosome-Derived ADAM17 Promotes Liver Metastasis in Colorectal Cancer
Abstract
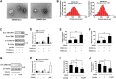
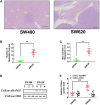
Exosomes derived from cancer cells are deemed important drivers of pre-metastatic niche formation at distant organs, but the underlying mechanisms of their effects remain largely unknow. Although the role of ADAM17 in cancer cells has been well studied, the secreted ADAM17 effects transported via exosomes are less understood. Herein, we show that the level of exosome-derived ADAM17 is elevated in the serum of patients with metastatic colorectal cancer as well as in metastatic colorectal cancer cells. Furthermore, exosomal ADAM17 was shown to promote the migratory ability of colorectal cancer cells by cleaving the E-cadherin junction. Moreover, exosomal ADAM17 overexpression as well as RNA interference results highlighted its function as a tumor metastasis-promoting factor in colorectal cancer in vitro and in vivo. Taken together, our current work suggests that exosomal ADAM17 is involved in pre-metastatic niche formation and may be utilized as a blood-based biomarker of colorectal cancer metastasis.
Keywords: ADAM17; E-cadherin; colorectal cancer; exosome; liver metastasis.
Copyright © 2021 Sun, Lu, Fu, Lu, Gu, Xu, Dai, Yang and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Tumor-derived exosomal ADAM17 promotes pre-metastatic niche formation by enhancing vascular permeability in colorectal cancer.J Exp Clin Cancer Res. 2024 Feb 27;43(1):59. doi: 10.1186/s13046-024-02991-3. J Exp Clin Cancer Res. 2024. PMID: 38413999 Free PMC article.
-
Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer.J Hematol Oncol. 2020 Nov 19;13(1):156. doi: 10.1186/s13045-020-00991-2. J Hematol Oncol. 2020. PMID: 33213490 Free PMC article.
-
Relation between Tetraspanin- Associated and Tetraspanin- Non- Associated Exosomal Proteases and Metabolic Syndrome in Colorectal Cancer Patients.Asian Pac J Cancer Prev. 2019 Mar 26;20(3):809-815. doi: 10.31557/APJCP.2019.20.3.809. Asian Pac J Cancer Prev. 2019. PMID: 30909692 Free PMC article.
-
A Snapshot of The Tumor Microenvironment in Colorectal Cancer: The Liquid Biopsy.Int J Mol Sci. 2019 Nov 29;20(23):6016. doi: 10.3390/ijms20236016. Int J Mol Sci. 2019. PMID: 31795332 Free PMC article. Review.
-
Exosomal noncoding RNAs in colorectal cancer.Cancer Lett. 2020 Nov 28;493:228-235. doi: 10.1016/j.canlet.2020.08.037. Epub 2020 Sep 6. Cancer Lett. 2020. PMID: 32898600 Review.
Cited by
-
Neutrophil and Colorectal Cancer.Int J Mol Sci. 2024 Dec 24;26(1):6. doi: 10.3390/ijms26010006. Int J Mol Sci. 2024. PMID: 39795864 Free PMC article. Review.
-
Therapeutic potential of extracellular vesicles from diverse sources in cancer treatment.Eur J Med Res. 2024 Jun 28;29(1):350. doi: 10.1186/s40001-024-01937-x. Eur J Med Res. 2024. PMID: 38943222 Free PMC article. Review.
-
Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring.Mol Cancer. 2022 Jan 20;21(1):25. doi: 10.1186/s12943-022-01505-z. Mol Cancer. 2022. PMID: 35057806 Free PMC article. Review.
-
Prognostic value of extracellular vesicles in colorectal cancer: a systematic review and meta-analysis.Clin Transl Oncol. 2025 Apr 9. doi: 10.1007/s12094-025-03915-z. Online ahead of print. Clin Transl Oncol. 2025. PMID: 40205153 Review.
-
Effects of Exosomes on Tumor Bioregulation and Diagnosis.ACS Omega. 2023 Jan 31;8(6):5157-5168. doi: 10.1021/acsomega.2c06567. eCollection 2023 Feb 14. ACS Omega. 2023. PMID: 36816660 Free PMC article. Review.
References
-
- Chafe S. C., Lou Y., Sceneay J., Vallejo M., Hamilton M. J., McDonald P. C., et al. (2015). Carbonic Anhydrase IX Promotes Myeloid-Derived Suppressor Cell Mobilization and Establishment of a Metastatic Niche by Stimulating G-CSF Production. Cancer Res. 75 (6), 996–1008. 10.1158/0008-5472.CAN-14-3000 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

