Pharmacy Compounded Medicines for Patients With Rare Diseases: Lessons Learned From Chenodeoxycholic Acid and Cholic Acid
- PMID: 34650439
- PMCID: PMC8505773
- DOI: 10.3389/fphar.2021.758210
Pharmacy Compounded Medicines for Patients With Rare Diseases: Lessons Learned From Chenodeoxycholic Acid and Cholic Acid
Abstract
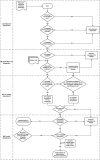
Patients with rare diseases are often confronted with the fact that effective medicines are unavailable or simply not being developed. This situation jeopardizes the health of a large population of vulnerable patients with rare diseases. Pharmacy compounded formulations can provide a safe alternative when authorized treatments are unavailable or unsuitable. Practical guidelines on how to develop and implement pharmacy compounded formulations for patients with rare diseases are limited. The aim of this article is to provide guidance for when and how to apply pharmacy compounded formulations for patients with rare diseases. This is illustrated with two challenging examples: the development and implementation of pharmacy compounding of 1) chenodeoxycholic acid (CDCA) capsules for patients with cerebrotendinous xanthomatosis (CTX) and 2) cholic acid (CA) capsules for patients with rare bile acid synthesis defects (BASD). All critical steps of the development of CDCA and CA capsules are explained and summarized in a practical guideline.
Keywords: bile acid synthesis defects; cerebrotendinous xanthomathosis; chenodeoxycholic acid; cholic acid; orphan medicines; pharmacy compounding; rare diseases.
Copyright © 2021 Polak, Jacobs and Kemper.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bouwman-Boer Y., Fenton-May V., Le Brun P. (2015b). “Practical Pharmaceutics,” in : Control Strategy Critical Quality Attributes, Process Parameters and Sources of Variability (Switzerland: Springer International Publishing; ). Chapter 17.7. 10.1007/978-3-319-15814-3 - DOI
-
- Bouwman-Boer Y., Fenton-May V., Le Brun P. (2015a). Practical Pharmaceutics. An International Guideline for the Preparation, Care and Use of Medicinal Products. Switzerland: Springer International Publishing.
-
- Bouwman-Boer Y., Fenton-May V., Le Brun P. (2015c). Practical Pharmaceutics. Chapter 17.8: Product Validation. Switzerland: Springer International Publishing.
LinkOut - more resources
Full Text Sources