Aortic Stiffness Hysteresis in Isolated Mouse Aortic Segments Is Intensified by Contractile Stimuli, Attenuated by Age, and Reversed by Elastin Degradation
- PMID: 34650441
- PMCID: PMC8507434
- DOI: 10.3389/fphys.2021.723972
Aortic Stiffness Hysteresis in Isolated Mouse Aortic Segments Is Intensified by Contractile Stimuli, Attenuated by Age, and Reversed by Elastin Degradation
Abstract
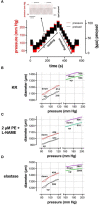
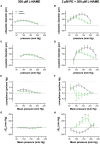
Aim: Cyclic stretch of vascular tissue at any given pressure reveals greater dimensions during unloading than during loading, which determines the cardiac beat-by-beat hysteresis loop on the pressure-diameter/volume relationship. The present study did not focus on hysteresis during a single stretch cycle but investigated whether aortic stiffness determined during continuous stretch at different pressures also displayed hysteresis phenomena. Methods: Aortic segments from C57Bl6 mice were mounted in the Rodent Oscillatory Set-up for Arterial Compliance (ROTSAC), where they were subjected to high frequency (10 Hz) cyclic stretch at alternating loads equivalent to a constant theoretical pulse pressure of 40 mm Hg. Diastolic and systolic diameter, compliance, and the Peterson elastic modulus (Ep), as a measure of aortic stiffness, was determined starting at cyclic stretch between alternating loads corresponding to 40 and 80 mm Hg, at each gradual load increase equivalent to 20 mm Hg, up to loads equivalent to pressures of 220 and 260 mm Hg (loading direction) and then repeated in the downward direction (unloading direction). This was performed in baseline conditions and following contraction by α1 adrenergic stimulation with phenylephrine or by depolarization with high extracellular K+ in aortas of young (5 months), aged (26 months) mice, and in segments treated with elastase. Results: In baseline conditions, diastolic/systolic diameters and compliance for a pulse pressure of 40 mm Hg were larger at any given pressure upon unloading (decreasing pressure) than loading (increasing pressure) of the aortic segments. The pressure-aortic stiffness (Ep) relationship was similar in the loading and unloading directions, and aortic hysteresis was absent. On the other hand, hysteresis was evident after activation of the VSMCs with the α1 adrenergic agonist phenylephrine and with depolarization by high extracellular K+, especially after inhibition of basal NO release with L-NAME. Aortic stiffness was significantly smaller in the unloading than in the loading direction. In comparison with young mice, old-mouse aortic segments also displayed contraction-dependent aortic hysteresis, but hysteresis was shifted to a lower pressure range. Elastase-treated segments showed higher stiffness upon unloading over nearly the whole pressure range. Conclusions: Mouse aortic segments display pressure- and contraction-dependent diameter, compliance, and stiffness hysteresis phenomena, which are modulated by age and VSMC-extracellular matrix interactions. This may have implications for aortic biomechanics in pathophysiological conditions and aging.
Keywords: aging; aortic stiffness; constriction; pressure-dependent hysteresis; viscosity.
Copyright © 2021 De Moudt, Leloup and Fransen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources

