DexA70, the Truncated Form of a Self-Produced Dextranase, Effectively Disrupts Streptococcus mutans Biofilm
- PMID: 34650538
- PMCID: PMC8505985
- DOI: 10.3389/fmicb.2021.737458
DexA70, the Truncated Form of a Self-Produced Dextranase, Effectively Disrupts Streptococcus mutans Biofilm
Abstract
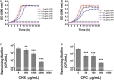
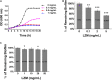
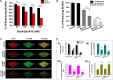
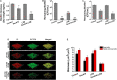
Billions of people suffer from dental caries every year in spite of the effort to reduce the prevalence over the past few decades. Streptococcus mutans is the leading member of a specific group of cariogenic bacteria that cause dental caries. S. mutans forms biofilm, which is highly resistant to harsh environment, host immunity, and antimicrobial treatments. In this study, we found that S. mutans biofilm is highly resistant to both antimicrobial agents and lysozyme. DexA70, the truncated form of DexA (amino acids 100-732), a dextranase in S. mutans, prevents S. mutans biofilm formation and disassembles existing biofilms within minutes at nanomolar concentrations when supplied exogenously. DexA70 treatment markedly enhances biofilm sensitivity to antimicrobial agents and lysozyme, indicating its great potential in combating biofilm-related dental caries.
Keywords: DexA70; Streptococcus mutans; biofilm; dental caries; lysozyme; polysaccharides.
Copyright © 2021 Liu, Li, Wang, Zhang, Wang, Zhang, Wang, Xu, Hu and Gu.
Conflict of interest statement
The authors have filed a patent application on the use of DexA70.
Figures

Similar articles
-
Effects of Antimicrobial Peptide GH12 on the Cariogenic Properties and Composition of a Cariogenic Multispecies Biofilm.Appl Environ Microbiol. 2018 Nov 30;84(24):e01423-18. doi: 10.1128/AEM.01423-18. Print 2018 Dec 15. Appl Environ Microbiol. 2018. PMID: 30341079 Free PMC article.
-
Regulation of water-soluble glucan synthesis by the Streptococcus mutans dexA gene effects biofilm aggregation and cariogenic pathogenicity.Mol Oral Microbiol. 2019 Apr;34(2):51-63. doi: 10.1111/omi.12253. Epub 2019 Feb 14. Mol Oral Microbiol. 2019. PMID: 30659765
-
Competition and Caries on Enamel of a Dual-Species Biofilm Model with Streptococcus mutans and Streptococcus sanguinis.Appl Environ Microbiol. 2020 Oct 15;86(21):e01262-20. doi: 10.1128/AEM.01262-20. Print 2020 Oct 15. Appl Environ Microbiol. 2020. PMID: 32826216 Free PMC article.
-
Streptococcus M utans-Specific Antimicrobial Peptide C16G2-Mediated Caries Prevention: A Review.Front Dent. 2022 Jun 28;19:17. doi: 10.18502/fid.v19i17.9963. eCollection 2022. Front Dent. 2022. PMID: 36458273 Free PMC article. Review.
-
[Secondary metabolites from Streptococcus mutans and their ecological roles in dental biofilm].Sheng Wu Gong Cheng Xue Bao. 2017 Sep 25;33(9):1547-1554. doi: 10.13345/j.cjb.170046. Sheng Wu Gong Cheng Xue Bao. 2017. PMID: 28956400 Review. Chinese.
Cited by
-
Microbiota Profiles of Hen Eggs from the Different Seasons and Different Sectors of Shanghai, China.Microorganisms. 2023 Oct 9;11(10):2519. doi: 10.3390/microorganisms11102519. Microorganisms. 2023. PMID: 37894177 Free PMC article.
-
Effect of mutanase and dextranase on biofilms of cariogenic bacteria: A systematic review of in vitro studies.Biofilm. 2024 May 22;7:100202. doi: 10.1016/j.bioflm.2024.100202. eCollection 2024 Jun. Biofilm. 2024. PMID: 38846328 Free PMC article. Review.
-
Dextranase enhances nanoparticle penetration of S. mutans biofilms.J Oral Microbiol. 2025 Jul 15;17(1):2528561. doi: 10.1080/20002297.2025.2528561. eCollection 2025. J Oral Microbiol. 2025. PMID: 40672866 Free PMC article.
-
Anti-Biofilm Activity of Chlorogenic Acid against Pseudomonas Using Quorum Sensing System.Foods. 2023 Sep 28;12(19):3601. doi: 10.3390/foods12193601. Foods. 2023. PMID: 37835254 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

