Oral Immunotherapy for Food-Allergic Children: A Pro-Con Debate
- PMID: 34650547
- PMCID: PMC8507468
- DOI: 10.3389/fimmu.2021.636612
Oral Immunotherapy for Food-Allergic Children: A Pro-Con Debate
Abstract
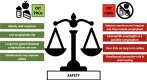
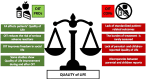
The prevalence of food allergy has increased in recent years, especially in children. Allergen avoidance, and drugs in case of an allergic reaction, remains the standard of care in food allergy. Nevertheless, increasing attention has been given to the possibility to treat food allergy, through immunotherapy, particularly oral immunotherapy (OIT). Several OIT protocols and clinical trials have been published. Most of them focus on children allergic to milk, egg, or peanut, although recent studies developed protocols for other foods, such as wheat and different nuts. OIT efficacy in randomized controlled trials is usually evaluated as the possibility for patients to achieve desensitization through the consumption of an increasing amount of a food allergen, while the issue of a possible long-term sustained unresponsiveness has not been completely addressed. Here, we evaluated current pediatric OIT knowledge, focusing on the results of clinical trials and current guidelines. Specifically, we wanted to highlight what is known in terms of OIT efficacy and effectiveness, safety, and impact on quality of life. For each aspect, we reported the pros and the cons, inferable from published literature. In conclusion, even though many protocols, reviews and meta-analysis have been published on this topic, pediatric OIT remains a controversial therapy and no definitive generalized conclusion may be drawn so far. It should be an option provided by specialized teams, when both patients and their families are prone to adhere to the proposed protocol. Efficacy, long-term effectiveness, possible role of adjuvant therapies, risk of severe reactions including anaphylaxis or eosinophilic esophagitis, and impact on the quality of life of both children and caregivers are all aspects that should be discussed before starting OIT. Future studies are needed to provide firm clinical and scientific evidence, which should also consider patient reported outcomes.
Keywords: IgE; anaphylaxis; food allergy; oral immunotherapy; pediatrics; reaction.
Copyright © 2021 Mori, Giovannini, Barni, Jiménez-Saiz, Munblit, Biagioni, Liccioli, Sarti, Liotti, Ricci, Novembre, Sahiner, Baldo and Caimmi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. . Worldwide Time Trends in the Prevalence of Symptoms of Asthma, Allergic Rhinoconjunctivitis, and Eczema in Childhood: ISAAC Phases One and Three Repeat Multicountry Cross-Sectional Surveys. Lancet (2006) 368(9537):733–43. doi: 10.1016/S0140-6736(06)69283-0 - DOI - PubMed
-
- Harrison FC, Giovannini M, Kalaichandran A, Santos AF. Food Allergy. eLS (2020), 1–12. doi: 10.1002/9780470015902.a0028380 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

