Advances in Adoptive Cell Therapy Using Induced Pluripotent Stem Cell-Derived T Cells
- PMID: 34650571
- PMCID: PMC8505955
- DOI: 10.3389/fimmu.2021.759558
Advances in Adoptive Cell Therapy Using Induced Pluripotent Stem Cell-Derived T Cells
Abstract
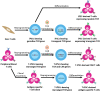
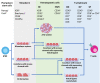
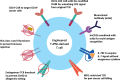
Adoptive cell therapy (ACT) using chimeric antigen receptor (CAR) T cells holds impressive clinical outcomes especially in patients who are refractory to other kinds of therapy. However, many challenges hinder its clinical applications. For example, patients who undergo chemotherapy usually have an insufficient number of autologous T cells due to lymphopenia. Long-term ex vivo expansion can result in T cell exhaustion, which reduces the effector function. There is also a batch-to-batch variation during the manufacturing process, making it difficult to standardize and validate the cell products. In addition, the process is labor-intensive and costly. Generation of universal off-the-shelf CAR T cells, which can be broadly given to any patient, prepared in advance and ready to use, would be ideal and more cost-effective. Human induced pluripotent stem cells (iPSCs) provide a renewable source of cells that can be genetically engineered and differentiated into immune cells with enhanced anti-tumor cytotoxicity. This review describes basic knowledge of T cell biology, applications in ACT, the use of iPSCs as a new source of T cells and current differentiation strategies used to generate T cells as well as recent advances in genome engineering to produce next-generation off-the-shelf T cells with improved effector functions. We also discuss challenges in the field and future perspectives toward the final universal off-the-shelf immunotherapeutic products.
Keywords: T cells; adoptive cell therapy; cancer immunotherapy; chimeric antigen receptor; induced pluripotent stem cells; off-the-shelf T cells; tumor infiltrating lymphocytes.
Copyright © 2021 Netsrithong and Wattanapanitch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

