Comparative Analyses of Phyllosphere Bacterial Communities and Metabolomes in Newly Developed Needles of Cunninghamia lanceolata (Lamb.) Hook. at Four Stages of Stand Growth
- PMID: 34650578
- PMCID: PMC8505725
- DOI: 10.3389/fpls.2021.717643
Comparative Analyses of Phyllosphere Bacterial Communities and Metabolomes in Newly Developed Needles of Cunninghamia lanceolata (Lamb.) Hook. at Four Stages of Stand Growth
Abstract
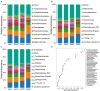
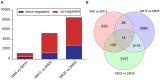
Host-plant-associated bacteria affect the growth, vigor, and nutrient availability of the host plant. However, phyllosphere bacteria have received less research attention and their functions remain elusive, especially in forest ecosystems. In this study, we collected newly developed needles from sapling (age 5 years), juvenile (15 years), mature (25 years), and overmature (35 years) stands of Chinese fir [Cunninghamia lanceolata (Lamb.) Hook]. We analyzed changes in phyllosphere bacterial communities, their functional genes, and metabolic activity among different stand ages. The results showed that phyllosphere bacterial communities changed, both in relative abundance and in composition, with an increase in stand age. Community abundance predominantly changed in the orders Campylobacterales, Pseudonocardiales, Deinococcales, Gemmatimonadales, Betaproteobacteriales, Chthoniobacterales, and Propionibacteriales. Functional predictions indicated the genes of microbial communities for carbon metabolism, nitrogen metabolism, antibiotic biosynthesis, flavonoids biosynthesis, and steroid hormone biosynthesis varied; some bacteria were strongly correlated with some metabolites. A total of 112 differential metabolites, including lipids, benzenoids, and flavonoids, were identified. Trigonelline, proline, leucine, and phenylalanine concentrations increased with stand age. Flavonoids concentrations were higher in sapling stands than in other stands, but the transcript levels of genes associated with flavonoids biosynthesis in the newly developed needles of saplings were lower than those of other stands. The nutritional requirements and competition between individual trees at different growth stages shaped the phyllosphere bacterial community and host-bacteria interaction. Gene expression related to the secondary metabolism of shikimate, mevalonate, terpenoids, tocopherol, phenylpropanoids, phenols, alkaloids, carotenoids, betains, wax, and flavonoids pathways were clearly different in Chinese fir at different ages. This study provides an overview of phyllosphere bacteria, metabolism, and transcriptome in Chinese fir of different stand ages and highlights the value of an integrated approach to understand the molecular mechanisms associated with biosynthesis.
Keywords: Cunninghamia lanceolata; functional genes; host-bacteria interaction; metabolic profile; phyllosphere bacterial community.
Copyright © 2021 Sun, Sun, Qiu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Ashihara H., Ludwig I. A., Katahira R., Yokota T., Fujimura T., Crozier A. (2015). Trigonelline and related nicotinic acid metabolites: occurrence, biosynthesis, taxonomic considerations, and their roles in planta and in human health. Phytochem. Rev. 14, 765–798. 10.1007/s11101-014-9375-z - DOI
LinkOut - more resources
Full Text Sources

