Bevacizumab Combined with Corticosteroids Does Not Improve the Clinical Outcome of Nasopharyngeal Carcinoma Patients With Radiation-Induced Brain Necrosis
- PMID: 34650930
- PMCID: PMC8506029
- DOI: 10.3389/fonc.2021.746941
Bevacizumab Combined with Corticosteroids Does Not Improve the Clinical Outcome of Nasopharyngeal Carcinoma Patients With Radiation-Induced Brain Necrosis
Abstract
Objective: Our aim was to compare the clinical outcomes of patients treated with bevacizumab combined with corticosteroids and those with bevacizumab monotherapy from a radiation-induced brain necrosis (RN) registry cohort (NCT03908502).
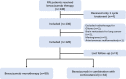
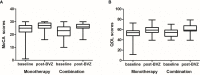
Methods: We utilized clinical data from a prospective RN registry cohort (NCT03908502) from July 2017 to June 2020. Patients were considered eligible if they had symptomatic RN after radiotherapy for nasopharyngeal carcinoma (NPC) and received bevacizumab (5 mg/kg, two to four cycles) with a minimum follow-up time of 3 months. The primary outcome was a 2-month response rate determined by MRI and clinical symptoms. Secondary outcomes included quality of life [evaluated by the abbreviated World Health Organization Quality of Life (WHOQOL-BREF) questionnaire] and cognitive function (evaluated by the Montreal Cognitive Assessment scale) at 2 months, RN recurrence during follow-up, and adverse events.
Results: A total of 123 patients (34 in the combined therapy group and 89 in the monotherapy group) were enrolled in our study with a median follow-up time of 0.97 year [interquartile range (IQR) = 0.35-2.60 years]. The clinical efficacy of RN did not differ significantly between patients in these two groups [odds ratio (OR) = 1.642, 95%CI = 0.584-4.614, p = 0.347]. Furthermore, bevacizumab combined with corticosteroids did not reduce recurrence compared with bevacizumab monotherapy [hazard ratio (HR) = 1.329, 95%CI = 0.849-2.079, p = 0.213]. The most common adverse events of bevacizumab were hypertension (17.89%), followed by nosebleed (8.13%) and fatigue (8.13%). There was no difference in grade 2 or more severe adverse events between the two groups (p = 0.811).
Interpretation: Our results showed that the treatment strategy of combining bevacizumab with corticosteroids did not lead to better clinical outcomes for RN patients with a background of radiotherapy for nasopharyngeal carcinoma.
Keywords: bevacizumab combined with corticosteroid; bevacizumab monotherapy; nasopharyngeal carcinoma; radiation-induced brain necrosis; real-world data.
Copyright © 2021 Li, Rong, Hu, Yang, Lei, Wen, Yue, Huang, Chua, Li, Cai, He, Pan, Cheng, Pi, Xue, Xu and Tang.
Conflict of interest statement
MC reports personal fees from Astellas, Janssen, Bayer, Pfizer, MSD, personal fees and non-financial support from AstraZeneca, personal fees and grants from Ferring, personal fees and non-financial support from Varian, non-financial support from Decipher Biosciences, non-financial support from MedLever, and consults for immunoSCAPE Inc., outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical

