Inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 Replication by Hypertonic Saline Solution in Lung and Kidney Epithelial Cells
- PMID: 34651104
- PMCID: PMC8442612
- DOI: 10.1021/acsptsci.1c00080
Inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 Replication by Hypertonic Saline Solution in Lung and Kidney Epithelial Cells
Abstract
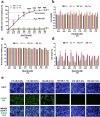
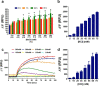
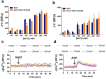
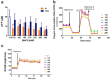
An unprecedented global health crisis has been caused by a new virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We performed experiments to test if a hypertonic saline solution was capable of inhibiting virus replication. Our data show that 1.2% NaCl inhibited virus replication by 90%, achieving 100% of inhibition at 1.5% in the nonhuman primate kidney cell line Vero, and 1.1% of NaCl was sufficient to inhibit the virus replication by 88% in human epithelial lung cell line Calu-3. Furthermore, our results indicate that the inhibition is due to an intracellular mechanism and not to the dissociation of the spike SARS-CoV-2 protein and its human receptor. NaCl depolarizes the plasma membrane causing a low energy state (high ADP/ATP concentration ratio) without impairing mitochondrial function, supposedly associated with the inhibition of the SARS-CoV-2 life cycle. Membrane depolarization and intracellular energy deprivation are possible mechanisms by which the hypertonic saline solution efficiently prevents virus replication in vitro assays.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Persistent replication of severe acute respiratory syndrome coronavirus in human tubular kidney cells selects for adaptive mutations in the membrane protein.J Virol. 2008 Jun;82(11):5137-44. doi: 10.1128/JVI.00096-08. Epub 2008 Mar 26. J Virol. 2008. PMID: 18367528 Free PMC article.
-
Replication of Severe Acute Respiratory Syndrome Coronavirus 2 in Human Respiratory Epithelium.J Virol. 2020 Jul 16;94(15):e00957-20. doi: 10.1128/JVI.00957-20. Print 2020 Jul 16. J Virol. 2020. PMID: 32434888 Free PMC article.
-
Suramin Inhibits SARS-CoV-2 Infection in Cell Culture by Interfering with Early Steps of the Replication Cycle.Antimicrob Agents Chemother. 2020 Jul 22;64(8):e00900-20. doi: 10.1128/AAC.00900-20. Print 2020 Jul 22. Antimicrob Agents Chemother. 2020. PMID: 32513797 Free PMC article.
-
SARS-CoV-2 Isolates Show Impaired Replication in Human Immune Cells but Differential Ability to Replicate and Induce Innate Immunity in Lung Epithelial Cells.Microbiol Spectr. 2021 Sep 3;9(1):e0077421. doi: 10.1128/Spectrum.00774-21. Epub 2021 Aug 11. Microbiol Spectr. 2021. PMID: 34378952 Free PMC article.
-
Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2.J Virol. 2010 Dec;84(24):12658-64. doi: 10.1128/JVI.01542-10. Epub 2010 Oct 6. J Virol. 2010. PMID: 20926566 Free PMC article.
Cited by
-
Early Negativization of SARS-CoV-2 Infection by Nasal Spray of Seawater plus Additives: The RENAISSANCE Open-Label Controlled Clinical Trial.Pharmaceutics. 2022 Nov 18;14(11):2502. doi: 10.3390/pharmaceutics14112502. Pharmaceutics. 2022. PMID: 36432693 Free PMC article.
-
Saline nasal irrigation and gargling in COVID-19: a multidisciplinary review of effects on viral load, mucosal dynamics, and patient outcomes.Front Public Health. 2023 Jun 16;11:1161881. doi: 10.3389/fpubh.2023.1161881. eCollection 2023. Front Public Health. 2023. PMID: 37397736 Free PMC article. Review.
-
Supporting the Aspecific Physiological Defenses of Upper Airways against Emerging SARS-CoV-2 Variants.Pathogens. 2023 Jan 29;12(2):211. doi: 10.3390/pathogens12020211. Pathogens. 2023. PMID: 36839483 Free PMC article.
-
A Hypertonic Seawater Nasal Irrigation Solution Containing Algal and Herbal Natural Ingredients Reduces Viral Load and SARS-CoV-2 Detection Time in the Nasal Cavity.J Pers Med. 2023 Jul 3;13(7):1093. doi: 10.3390/jpm13071093. J Pers Med. 2023. PMID: 37511706 Free PMC article.
-
In vitro testing of salt coating of fabrics as a potential antiviral agent in reusable face masks.Sci Rep. 2022 Oct 11;12(1):17041. doi: 10.1038/s41598-022-21442-7. Sci Rep. 2022. PMID: 36220878 Free PMC article.
References
-
- Zhu N.; Zhang D.; Wang W.; Li X.; Yang B.; Song J.; Zhao X.; Huang B.; Shi W.; Lu R.; Niu P.; Zhan F.; Ma X.; Wang D.; Xu W.; Wu G.; Gao G. F.; Tan W. China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382 (8), 727–733. 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Wang K.; Chen W.; Zhang Z.; Deng Y.; Lian J.-Q.; Du P.; Wei D.; Zhang Y.; Sun X.-X.; Gong L.; Yang X.; He L.; Zhang L.; Yang Z.; Geng J.-J.; Chen R.; Zhang H.; Wang B.; Zhu Y.-M.; Nan G.; Jiang J.-L.; Li L.; Wu J.; Lin P.; Huang W.; Xie L.; Zheng Z.-H.; Zhang K.; Miao J.-L.; Cui H.-Y.; Huang M.; Zhang J.; Fu L.; Yang X.-M.; Zhao Z.; Sun S.; Gu H.; Wang Z.; Wang C.-F.; Lu Y.; Liu Y.-Y.; Wang Q.-Y.; Bian H.; Zhu P.; Chen Z.-N. CD147-Spike Protein Is a Novel Route for SARS-CoV-2 Infection to Host Cells. Signal Transduct Target Ther 2020, 5 (1), 283. 10.1038/s41392-020-00426-x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
