Allopregnanolone and neuroHIV: Potential benefits of neuroendocrine modulation in the era of antiretroviral therapy
- PMID: 34651359
- PMCID: PMC8866218
- DOI: 10.1111/jne.13047
Allopregnanolone and neuroHIV: Potential benefits of neuroendocrine modulation in the era of antiretroviral therapy
Abstract
Forty years into the HIV pandemic, approximately 50% of infected individuals still suffer from a constellation of neurological disorders collectively known as 'neuroHIV.' Although combination antiretroviral therapy (cART) has been a tremendous success, in its present form, it cannot eradicate HIV. Reservoirs of virus reside within the central nervous system, serving as sources of HIV virotoxins that damage mitochondria and promote neurotoxicity. Although understudied, there is evidence that HIV or the HIV regulatory protein, trans-activator of transcription (Tat), can dysregulate neurosteroid formation potentially contributing to endocrine dysfunction. People living with HIV commonly suffer from endocrine disorders, including hypercortisolemia accompanied by paradoxical adrenal insufficiency upon stress. Age-related comorbidities often onset sooner and with greater magnitude among people living with HIV and are commonly accompanied by hypogonadism. In the post-cART era, these derangements of the hypothalamic-pituitary-adrenal and -gonadal axes are secondary (i.e., relegated to the brain) and indicative of neuroendocrine dysfunction. We review the clinical and preclinical evidence for neuroendocrine dysfunction in HIV, the capacity for hormone therapeutics to play an ameliorative role and the future steroid-based therapeutics that may have efficacy as novel adjunctives to cART.
Keywords: HIV-associated neurocognitive disorder; hypothalamic-pituitary-adrenal axis; hypothalamic-pituitary-gonadal axis; mitochondria; neurosteroids.
© 2021 British Society for Neuroendocrinology.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
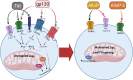

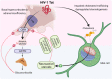

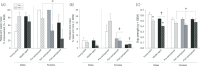
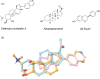
References
-
- Global HIV and AIDS Statistics‐Fact Sheet . Preliminary UNAIDS 2021 Epidemiological Estimates. https://www.unaids.org/en/resources/fact‐sheet. Accessed June 6, 2021.
-
- Zink WE, Zheng J, Persidsky Y, Poluektova L, Gendelman HE. The neuropathogenesis of HIV‐1 infection. FEMS Immunol Med Microbiol. 1999;26(3–4):233‐241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

