Urinary extracellular vesicles: Assessment of pre-analytical variables and development of a quality control with focus on transcriptomic biomarker research
- PMID: 34651466
- PMCID: PMC8517090
- DOI: 10.1002/jev2.12158
Urinary extracellular vesicles: Assessment of pre-analytical variables and development of a quality control with focus on transcriptomic biomarker research
Abstract
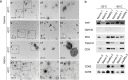
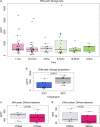
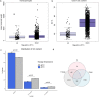
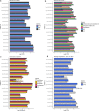
Urinary extracellular vesicles (uEV) are a topical source of non-invasive biomarkers for health and diseases of the urogenital system. However, several challenges have become evident in the standardization of uEV pipelines from collection of urine to biomarker analysis. Here, we studied the effect of pre-analytical variables and developed means of quality control for uEV isolates to be used in transcriptomic biomarker research. We included urine samples from healthy controls and individuals with type 1 or type 2 diabetes and normo-, micro- or macroalbuminuria and isolated uEV by ultracentrifugation. We studied the effect of storage temperature (-20°C vs. -80°C), time (up to 4 years) and storage format (urine or isolated uEV) on quality of uEV by nanoparticle tracking analysis, electron microscopy, Western blotting and qPCR. Urinary EV RNA was compared in terms of quantity, quality, and by mRNA or miRNA sequencing. To study the stability of miRNA levels in samples isolated by different methods, we created and tested a list of miRNAs commonly enriched in uEV isolates. uEV and their transcriptome were preserved in urine or as isolated uEV even after long-term storage at -80°C. However, storage at -20°C degraded particularly the GC-rich part of the transcriptome and EV protein markers. Transcriptome was preserved in RNA samples extracted with and without DNAse, but read distributions still showed some differences in e.g. intergenic and intronic reads. MiRNAs commonly enriched in uEV isolates were stable and concordant between different EV isolation methods. Analysis of never frozen uEV helped to identify surface characteristics of particles by EM. In addition to uEV, qPCR assays demonstrated that uEV isolates commonly contained polyoma viruses. Based on our results, we present recommendations how to store and handle uEV isolates for transcriptomics studies that may help to expedite standardization of the EV biomarker field.
Keywords: DNAse; biomarkers; mRNA; microRNA; storage temperature; storage time; transcriptomics; urinary exosomes; urinary extracellular vesicles; virus.
© 2021 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
KB, OPD, SV, CF, PG, TT, HH, MY, PS, ES, TH, SL, AR, LG, MP, have no conflict of interest to declare.
Figures








References
-
- Adiconis, X. , Borges‐Rivera, D. , Satija, R. , Deluca, D. S. , Busby, M. A. , Berlin, A. M. , Sivachenko, A. , Thompson, D. A. , Wysoker, A. , Fennell, T. , Gnirke, A. , Pochet, N. , Regev, A. , & Levin, J. Z. (2013). Comparative analysis of RNA sequencing methods for degraded or low‐input samples. Nature Methods, 10(7), 623–629. 10.1038/nmeth.2483 - DOI - PMC - PubMed
-
- Armstrong, D. A. , Dessaint, J. A. , Ringelberg, C. S. , Hazlett, H. F. , Howard, L. , Abdalla, M. A.K. , Barnaby, R. L. , Stanton, B. A. , Cervinski, M. A. , & Ashare, A. (2018). Pre‐analytical handling conditions and small RNA recovery from urine for miRNA profiling. The Journal of Molecular Diagnostics, 20(5), 565–571. 10.1016/j.jmoldx.2018.04.003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

