Temporal manipulation of Cdkl5 reveals essential postdevelopmental functions and reversible CDKL5 deficiency disorder-related deficits
- PMID: 34651584
- PMCID: PMC8516470
- DOI: 10.1172/JCI143655
Temporal manipulation of Cdkl5 reveals essential postdevelopmental functions and reversible CDKL5 deficiency disorder-related deficits
Abstract
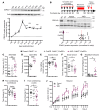
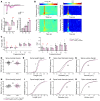
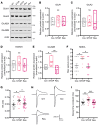
CDKL5 deficiency disorder (CDD) is an early onset, neurodevelopmental syndrome associated with pathogenic variants in the X-linked gene encoding cyclin-dependent kinase-like 5 (CDKL5). CDKL5 has been implicated in neuronal synapse maturation, yet its postdevelopmental necessity and the reversibility of CDD-associated impairments remain unknown. We temporally manipulated endogenous Cdkl5 expression in male mice and found that postdevelopmental loss of CDKL5 disrupts numerous behavioral domains, hippocampal circuit communication, and dendritic spine morphology, demonstrating an indispensable role for CDKL5 in the adult brain. Accordingly, restoration of Cdkl5 after the early stages of brain development using a conditional rescue mouse model ameliorated CDD-related behavioral impairments and aberrant NMDA receptor signaling. These findings highlight the requirement of CDKL5 beyond early development, underscore the potential for disease reversal in CDD, and suggest that a broad therapeutic time window exists for potential treatment of CDD-related deficits.
Keywords: Development; Genetic diseases; Neurological disorders; Neuroscience; Synapses.
Conflict of interest statement
Figures

Comment in
-
CDKL5 deficiency disorder: a pathophysiology of neural maintenance.J Clin Invest. 2021 Nov 1;131(21):e153606. doi: 10.1172/JCI153606. J Clin Invest. 2021. PMID: 34720088 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

