Synthesis and grafting of diazonium tosylates for thermoplastic electrode immunosensors
- PMID: 34651620
- PMCID: PMC8628260
- DOI: 10.1039/d1ay00965f
Synthesis and grafting of diazonium tosylates for thermoplastic electrode immunosensors
Abstract
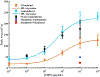
For electrochemical immunosensors, inexpensive electrodes with fast redox kinetics, and simple stable methods of electrode functionalization are vital. However, many inexpensive and easy to fabricate electrodes suffer from poor redox kinetics, and functionalization can often be difficult and/or unstable. Diazonium tosylates are particularly stable soluble salts that can be useful for electrode functionalization. Recently developed thermoplastic electrodes (TPEs) have been inexpensive, moldable, and highly electroactive carbon composite materials. Herein, the synthesis and grafting of diazonium tosylate salts were optimized for modification of TPEs and used to develop the first TPE immunosensors. With diazonium tosylates, TPEs were amine functionalized either directly through grafting of p-aminophenyl diazonium salt or indirectly through grafting p-nitrophenyl diazonium salt followed by electrochemical reduction to an amine. Diazonium tosylates were synthesized in situ as a paste in 6 min. Once the reaction paste was spread over the electrodes, near monolayer coverage (1.0 ± 0.2 nmol cm-2) was achieved for p-nitrophenyl diazonium salt within 5 min. Amine functionalized electrodes were conjugated to C-reactive protein (CRP) antibodies. Antibody-modified TPEs were applied for the sensitive detection of CRP, a biomarker of cardiovascular disease using electrochemical enzyme-linked immunosorbent assays (ELISA). LODs were determined to be 2 ng mL-1 in buffer, with high selectivity against interfering species for both functionalization methods. The direct p-aminophenyl modification method had the highest sensitivity to CRP and was further tested in spiked serum with an LOD of 10 ng mL-1. This low-cost and robust TPE immunosensor platform can be easily adapted for other analytes and multiplexed detection.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures





References
-
- Bahadir EB and Sezginturk MK, Talanta, 2015, 132, 162–174. - PubMed
-
- Heineman WR and Halsall HB, Anal. Chem, 1985, 57, 1321A–1331A. - PubMed
-
- Wehmeyer KR, Halsall H and Heineman W, W. Clin. Chem 1985, 31, 1546–1549. - PubMed
-
- McCreery RL, Chem. Rev, 2008, 108, 2646–2687. - PubMed
-
- Klunder KJ, Nilsson Z, Sambur JB and Henry CS, J. Am. Chem. Soc 2017, 139, 12623–12631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

