Catalytic Control of Spiroketal Formation in Rubromycin Polyketide Biosynthesis
- PMID: 34652045
- PMCID: PMC9299503
- DOI: 10.1002/anie.202109384
Catalytic Control of Spiroketal Formation in Rubromycin Polyketide Biosynthesis
Abstract
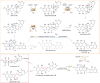
The medically important bacterial aromatic polyketide natural products typically feature a planar, polycyclic core structure. An exception is found for the rubromycins, whose backbones are disrupted by a bisbenzannulated [5,6]-spiroketal pharmacophore that was recently shown to be assembled by flavin-dependent enzymes. In particular, a flavoprotein monooxygenase proved critical for the drastic oxidative rearrangement of a pentangular precursor and the installment of an intermediate [6,6]-spiroketal moiety. Here we provide structural and mechanistic insights into the control of catalysis by this spiroketal synthase, which fulfills several important functions as reductase, monooxygenase, and presumably oxidase. The enzyme hereby tightly controls the redox state of the substrate to counteract shunt product formation, while also steering the cleavage of three carbon-carbon bonds. Our work illustrates an exceptional strategy for the biosynthesis of stable chroman spiroketals.
Keywords: antibiotics; collinone; lenticulone; polyketide synthase; redox tailoring.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Yunt Z., Reinhardt K., Li A., Engeser M., Dahse H.-M., Gütschow M., Bruhn T., Bringmann G., Piel J., J. Am. Chem. Soc. 2009, 131, 2297. - PubMed
-
- Li A., Piel J., Chem. Biol. 2002, 9, 1017. - PubMed
-
- Lackner G., Schenk A., Xu Z., Reinhardt K., Yunt Z. S., Piel J., Hertweck C., J. Am. Chem. Soc. 2007, 129, 9306. - PubMed
-
- Ueno T., Takahashi H., Oda M., Mizunuma M., Yokoyama A., Goto Y., Mizushina Y., Sakaguchi K., Hayashi H., Biochemistry 2000, 39, 5995. - PubMed
-
- Martin R., Sterner O., Alvarez M. A., de Clercq E., Bailey J. E., Minas W., J. Antibiot. 2001, 54, 239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

