Regulatory T-cells inhibit microglia-induced pain hypersensitivity in female mice
- PMID: 34652270
- PMCID: PMC8639143
- DOI: 10.7554/eLife.69056
Regulatory T-cells inhibit microglia-induced pain hypersensitivity in female mice
Abstract
Peripheral nerve injury-induced neuropathic pain is a chronic and debilitating condition characterized by mechanical hypersensitivity. We previously identified microglial activation via release of colony-stimulating factor 1 (CSF1) from injured sensory neurons as a mechanism contributing to nerve injury-induced pain. Here, we show that intrathecal administration of CSF1, even in the absence of injury, is sufficient to induce pain behavior, but only in male mice. Transcriptional profiling and morphologic analyses after intrathecal CSF1 showed robust immune activation in male but not female microglia. CSF1 also induced marked expansion of lymphocytes within the spinal cord meninges, with preferential expansion of regulatory T-cells (Tregs) in female mice. Consistent with the hypothesis that Tregs actively suppress microglial activation in females, Treg deficient (Foxp3DTR) female mice showed increased CSF1-induced microglial activation and pain hypersensitivity equivalent to males. We conclude that sexual dimorphism in the contribution of microglia to pain results from Treg-mediated suppression of microglial activation and pain hypersensitivity in female mice.
Keywords: CSF1; Treg; meninges; microglia; mouse; neuroscience; pain; spinal cord.
© 2021, Kuhn et al.
Conflict of interest statement
JK Patent approved on use of CSF1 blockade to treat neuropathic pain (Publication Number WO/2016/057800). IV, JB, KH, MB, VC, MD, JO, AM, AM No competing interests declared, AB Reviewing editor, eLife
Figures
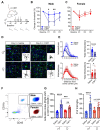
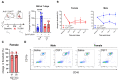

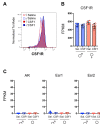

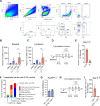

Comment in
-
Why sex matters.Elife. 2021 Dec 2;10:e74935. doi: 10.7554/eLife.74935. Elife. 2021. PMID: 34854810 Free PMC article.
References
-
- Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, Taravati K, Tan MR, Ricardo-Gonzalez RR, Nosbaum A, Bertolini M, Liao W, Nestle FO, Paus R, Cotsarelis G, Abbas AK, Rosenblum MD. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell. 2017;169:1119–1129. doi: 10.1016/j.cell.2017.05.002. - DOI - PMC - PubMed
-
- Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. The Journal of Neuroscience. 2009;29:14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

