Activation of the XBP1s/O-GlcNAcylation Pathway Improves Functional Outcome After Cardiac Arrest and Resuscitation in Young and Aged Mice
- PMID: 34652341
- PMCID: PMC9059164
- DOI: 10.1097/SHK.0000000000001732
Activation of the XBP1s/O-GlcNAcylation Pathway Improves Functional Outcome After Cardiac Arrest and Resuscitation in Young and Aged Mice
Abstract
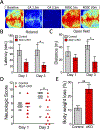
After cardiac arrest (CA) and resuscitation, the unfolded protein response (UPR) is activated in various organs including the brain. However, the role of the UPR in CA outcome remains largely unknown. One UPR branch involves spliced X-box-binding protein-1 (XBP1s). Notably, XBP1s, a transcriptional factor, can upregulate expression of specific enzymes related to glucose metabolism, and subsequently boost O-linked β-N-acetylglucosamine modification (O-GlcNAcylation). The current study is focused on effects of the XBP1 UPR branch and its downstream O-GlcNAcylation on CA outcome. Using both loss-of-function and gain-of-function mouse genetic tools, we provide the first evidence that activation of the XBP1 UPR branch in the post-CA brain is neuroprotective. Specifically, neuron-specific Xbp1 knockout mice had worse CA outcome, while mice with neuron-specific expression of Xbp1s in the brain had better CA outcome. Since it has been shown that the protective role of the XBP1s signaling pathway under ischemic conditions is mediated by increasing O-GlcNAcylation, we then treated young mice with glucosamine, and found that functional deficits were mitigated on day 3 post CA. Finally, after confirming that glucosamine can boost O-GlcNAcylation in the aged brain, we subjected aged mice to 8 min CA, and then treated them with glucosamine. We found that glucosamine-treated aged mice performed significantly better in behavioral tests. Together, our data indicate that the XBP1s/O-GlcNAc pathway is a promising target for CA therapy.
Copyright © 2021 by the Shock Society.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

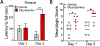


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

