Sorbitol Is a Severity Biomarker for PMM2-CDG with Therapeutic Implications
- PMID: 34652821
- PMCID: PMC8820356
- DOI: 10.1002/ana.26245
Sorbitol Is a Severity Biomarker for PMM2-CDG with Therapeutic Implications
Abstract
Objective: Epalrestat, an aldose reductase inhibitor increases phosphomannomutase (PMM) enzyme activity in a PMM2-congenital disorders of glycosylation (CDG) worm model. Epalrestat also decreases sorbitol level in diabetic neuropathy. We evaluated the genetic, biochemical, and clinical characteristics, including the Nijmegen Progression CDG Rating Scale (NPCRS), urine polyol levels and fibroblast glycoproteomics in patients with PMM2-CDG.
Methods: We performed PMM enzyme measurements, multiplexed proteomics, and glycoproteomics in PMM2-deficient fibroblasts before and after epalrestat treatment. Safety and efficacy of 0.8 mg/kg/day oral epalrestat were studied in a child with PMM2-CDG for 12 months.
Results: PMM enzyme activity increased post-epalrestat treatment. Compared with controls, 24% of glycopeptides had reduced abundance in PMM2-deficient fibroblasts, 46% of which improved upon treatment. Total protein N-glycosylation improved upon epalrestat treatment bringing overall glycosylation toward the control fibroblasts' glycosylation profile. Sorbitol levels were increased in the urine of 74% of patients with PMM2-CDG and correlated with the presence of peripheral neuropathy, and CDG severity rating scale. In the child with PMM2-CDG on epalrestat treatment, ataxia scores improved together with significant growth improvement. Urinary sorbitol levels nearly normalized in 3 months and blood transferrin glycosylation normalized in 6 months.
Interpretation: Epalrestat improved PMM enzyme activity, N-glycosylation, and glycosylation biomarkers in vitro. Leveraging cellular glycoproteome assessment, we provided a systems-level view of treatment efficacy and discovered potential novel biosignatures of therapy response. Epalrestat was well-tolerated and led to significant clinical improvements in the first pediatric patient with PMM2-CDG treated with epalrestat. We also propose urinary sorbitol as a novel biomarker for disease severity and treatment response in future clinical trials in PMM2-CDG. ANN NEUROL 20219999:n/a-n/a.
© 2021 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest
Mayo Clinic and Eva Morava have a financial interest related to this research. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies. Maggie’s Pearl LLC and Ethan Perlstein have a financial interest related to this research. Eva Morava and Mayo Clinic has a “Know How” on epalrestat treatment development for future clinical trials entitled
Figures



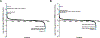




References
-
- Altassan R, Péanne R, Jaeken J, et al. International clinical guidelines for the management of phosphomannomutase 2-congenital disorders of glycosylation: diagnosis, treatment and follow up. J Inherit Metab Dis 2019;42:5–28. - PubMed
-
- Witters P, Honzik T, Bauchart E, et al. Long-term follow-up in PMM2-CDG: are we ready to start treatment trials? Genet Med 2019;21:1181–1188. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

