Receptor binding domain-IgG levels correlate with protection in residents facing SARS-CoV-2 B.1.1.7 outbreaks
- PMID: 34652831
- PMCID: PMC8652754
- DOI: 10.1111/all.15142
Receptor binding domain-IgG levels correlate with protection in residents facing SARS-CoV-2 B.1.1.7 outbreaks
Abstract
Background: Limited information exists on nursing home (NH) residents regarding BNT162b2 vaccine efficacy in preventing SARS-CoV-2 and severe COVID-19, and its association with post-vaccine humoral response.
Methods: 396 residents from seven NHs suffering a SARS-CoV-2 B.1.1.7 (VOC-α) outbreak at least 14 days after a vaccine campaign were repeatedly tested using SARS-CoV-2 real-time reverse-transcriptase polymerase chain reaction on nasopharyngeal swab test (RT-qPCR). SARS-CoV-2 receptor-binding domain (RBD) of the S1 subunit (RBD-IgG) was measured in all residents. Nucleocapsid antigenemia (N-Ag) was measured in RT-qPCR-positive residents and serum neutralizing antibodies in vaccinated residents from one NH.
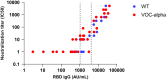
Results: The incidence of positive RT-qPCR was lower in residents vaccinated by two doses (72/317; 22.7%) vs one dose (10/31; 32.3%) or non-vaccinated residents (21/48; 43.7%; p < .01). COVID-19-induced deaths were observed in 5 of the 48 non-vaccinated residents (10.4%), in 2 of the 31 who had received one dose (6.4%), and in 3 of the 317 (0.9%) who had received two doses (p = .0007). Severe symptoms were more common in infected non-vaccinated residents (10/21; 47.6%) than in infected vaccinated residents (15/72; 21.0%; p = .002). Higher levels of RBD-IgG (n = 325) were associated with a lower SARS-CoV-2 incidence. No in vitro serum neutralization activity was found for RBD-IgG levels below 1050 AU/ml. RBD-IgG levels were inversely associated with N-Ag levels, found as a risk factor of severe COVID-19.
Conclusions: Two BNT162b2 doses are associated with a 48% reduction of SARS-CoV-2 incidence and a 91.3% reduction of death risk in residents from NHs facing a VOC-α outbreak. Post-vaccine RBD-IgG levels correlate with BNT162b2 protection against SARS-CoV-2 B.1.1.7.
Keywords: BNT162b2 vaccine; COVID-19; SARS-CoV-2; antibody response; efficacy; neutralizing antibodies; nucleocapsid antigenemia; nursing homes; residents; symptoms.
© 2021 European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest/competing interests.
Figures
References
-
- Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer‐BioNTech and Moderna Vaccines in Preventing SARS‐CoV‐2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS‐CoV‐2 B.1.617.2 (Delta) Variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Weekly Report. 2021;70(34):1163–1166. doi:10.15585/mmwr.mm7034e3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

