Structural analysis of the boronic acid β-lactamase inhibitor vaborbactam binding to Pseudomonas aeruginosa penicillin-binding protein 3
- PMID: 34653211
- PMCID: PMC8519428
- DOI: 10.1371/journal.pone.0258359
Structural analysis of the boronic acid β-lactamase inhibitor vaborbactam binding to Pseudomonas aeruginosa penicillin-binding protein 3
Abstract
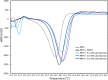
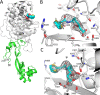
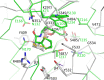
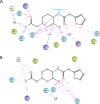
Antimicrobial resistance (AMR) mediated by β-lactamases is the major and leading cause of resistance to penicillins and cephalosporins among Gram-negative bacteria. β-Lactamases, periplasmic enzymes that are widely distributed in the bacterial world, protect penicillin-binding proteins (PBPs), the major cell wall synthesizing enzymes, from inactivation by β-lactam antibiotics. Developing novel PBP inhibitors with a non-β-lactam scaffold could potentially evade this resistance mechanism. Based on the structural similarities between the evolutionary related serine β-lactamases and PBPs, we investigated whether the potent β-lactamase inhibitor, vaborbactam, could also form an acyl-enzyme complex with Pseudomonas aeruginosa PBP3. We found that this cyclic boronate, vaborbactam, inhibited PBP3 (IC50 of 262 μM), and its binding to PBP3 increased the protein thermal stability by about 2°C. Crystallographic analysis of the PBP3:vaborbactam complex reveals that vaborbactam forms a covalent bond with the catalytic S294. The amide moiety of vaborbactam hydrogen bonds with N351 and the backbone oxygen of T487. The carboxyl group of vaborbactam hydrogen bonds with T487, S485, and S349. The thiophene ring and cyclic boronate ring of vaborbactam form hydrophobic interactions, including with V333 and Y503. The active site of the vaborbactam-bound PBP3 harbors the often observed ligand-induced formation of the aromatic wall and hydrophobic bridge, yet the residues involved in this wall and bridge display much higher temperature factors compared to PBP3 structures bound to high-affinity β-lactams. These insights could form the basis for developing more potent novel cyclic boronate-based PBP inhibitors to inhibit these targets and overcome β-lactamases-mediated resistance mechanisms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Decoding the Penicillin-Binding Proteins with Activity-Based Probes.Acc Chem Res. 2025 Jun 3;58(11):1754-1763. doi: 10.1021/acs.accounts.5c00113. Epub 2025 May 21. Acc Chem Res. 2025. PMID: 40396497 Review.
-
Specific variants in ftsI reduce carbapenem susceptibility in Pseudomonas aeruginosa.Microbiol Spectr. 2025 Aug 5;13(8):e0102725. doi: 10.1128/spectrum.01027-25. Epub 2025 Jul 7. Microbiol Spectr. 2025. PMID: 40621918 Free PMC article.
-
Structural Characterization of Diazabicyclooctane β-Lactam "Enhancers" in Complex with Penicillin-Binding Proteins PBP2 and PBP3 of Pseudomonas aeruginosa.mBio. 2021 Feb 16;12(1):e03058-20. doi: 10.1128/mBio.03058-20. mBio. 2021. PMID: 33593978 Free PMC article.
-
Design and Synthesis of Bimetallic Cu(II) Compounds as Potent Antibacterial and Antibiofilm Agents with Metallo-β-Lactamase Inhibitory Activity Against Multidrug Resistant Pseudomonas aeruginosa.Chemistry. 2025 Jul 22;31(41):e202501313. doi: 10.1002/chem.202501313. Epub 2025 Jul 4. Chemistry. 2025. PMID: 40534417
-
Metallo-β-lactamase inhibitors: A continuing challenge for combating antibiotic resistance.Biophys Chem. 2024 Jun;309:107228. doi: 10.1016/j.bpc.2024.107228. Epub 2024 Mar 25. Biophys Chem. 2024. PMID: 38552402 Review.
Cited by
-
Synthesis, Biological Properties, In Silico ADME, Molecular Docking Studies, and FMO Analysis of Chalcone Derivatives as Promising Antioxidant and Antimicrobial Agents.ACS Omega. 2025 Jan 28;10(5):4367-4387. doi: 10.1021/acsomega.4c06897. eCollection 2025 Feb 11. ACS Omega. 2025. PMID: 39959036 Free PMC article.
-
Structural Characterization of the D179N and D179Y Variants of KPC-2 β-Lactamase: Ω-Loop Destabilization as a Mechanism of Resistance to Ceftazidime-Avibactam.Antimicrob Agents Chemother. 2022 Apr 19;66(4):e0241421. doi: 10.1128/aac.02414-21. Epub 2022 Mar 28. Antimicrob Agents Chemother. 2022. PMID: 35341315 Free PMC article.
-
Exploring the inhibition of the soluble lytic transglycosylase Cj0843c of Campylobacter jejuni via targeting different sites with different scaffolds.Protein Sci. 2023 Jul;32(7):e4683. doi: 10.1002/pro.4683. Protein Sci. 2023. PMID: 37209283 Free PMC article.
-
Boronic acid inhibitors of penicillin-binding protein 1b: serine and lysine labelling agents.J Enzyme Inhib Med Chem. 2024 Dec;39(1):2305833. doi: 10.1080/14756366.2024.2305833. Epub 2024 Feb 27. J Enzyme Inhib Med Chem. 2024. PMID: 38410950 Free PMC article.
-
Repurposing Amphotericin B: anti-microbial, molecular docking and molecular dynamics simulation studies suggest inhibition potential of Amphotericin B against MRSA.BMC Chem. 2023 Jun 29;17(1):67. doi: 10.1186/s13065-023-00980-9. BMC Chem. 2023. PMID: 37386581 Free PMC article.
References
-
- Curtis NA, Orr D, Ross GW, Boulton MG. Competition of β-lactam antibiotics for the penicillin-binding proteins of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli: comparison with antibacterial activity and effects upon bacterial morphology. Antimicrobial agents and chemotherapy. 1979;16(3):325–8. doi: 10.1128/AAC.16.3.325 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

