Crinecerfont Lowers Elevated Hormone Markers in Adults With 21-Hydroxylase Deficiency Congenital Adrenal Hyperplasia
- PMID: 34653252
- PMCID: PMC8851935
- DOI: 10.1210/clinem/dgab749
Crinecerfont Lowers Elevated Hormone Markers in Adults With 21-Hydroxylase Deficiency Congenital Adrenal Hyperplasia
Abstract
Context: Classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency (21OHD) is characterized by impaired cortisol synthesis and excess androgen production. Corticotropin-releasing factor type 1 receptor (CRF1R) antagonism may decrease adrenal androgen production.
Objective: This work aimed to evaluate the safety, tolerability, and efficacy of crinecerfont (NBI-74788), a selective CRF1R antagonist, in 21OHD.
Methods: This open-label, phase 2 study, with sequential cohort design (NCT03525886), took place in 6 centers in the United States. Participants included men and women, aged 18 to 50 years, with 21OHD. Interventions included 4 crinecerfont regimens, each administered orally for 14 consecutive days: 50 or 100 mg once daily at bedtime (cohorts 1 and 2, respectively); 100 mg once daily in the evening (cohort 3); and 100 mg twice daily (cohort 4). Participants could enroll in more than 1 cohort. Main outcomes included changes from baseline to day 14 in adrenocorticotropin (ACTH), 17-hydroxyprogesterone (17OHP), androstenedione, and testosterone.
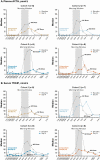
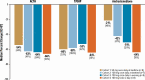
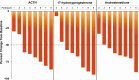
Results: Eighteen participants (11 women, 7 men) were enrolled: cohort 1 (n = 8), cohort 2 (n = 7), cohort 3 (n = 8), cohort 4 (n = 8). Mean age was 31 years; 94% were White. Median percent reductions were more than 60% for ACTH (-66%), 17OHP (-64%), and androstenedione (-64%) with crinecerfont 100 mg twice a day. In female participants, 73% (8/11) had a 50% or greater reduction in testosterone levels; male participants had median 26% to 65% decreases in androstenedione/testosterone ratios.
Conclusion: Crinecerfont treatment for 14 days lowered ACTH and afforded clinically meaningful reductions of elevated 17OHP, androstenedione, testosterone (women), or androstenedione/testosterone ratio (men) in adults with 21OHD. Longer-term studies are required to evaluate the effects of crinecerfont on clinical end points of disordered steroidogenesis and glucocorticoid exposure in patients with 21OHD.
Keywords: 17-hydroxyprogesterone; 21-hydroxylase deficiency; NBI-74788; congenital adrenal hyperplasia; crinecerfont.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365(9477):2125-2136. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

