Early assessment of circulating tumor DNA after curative-intent resection predicts tumor recurrence in early-stage and locally advanced non-small-cell lung cancer
- PMID: 34653314
- PMCID: PMC8763652
- DOI: 10.1002/1878-0261.13116
Early assessment of circulating tumor DNA after curative-intent resection predicts tumor recurrence in early-stage and locally advanced non-small-cell lung cancer
Abstract
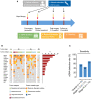
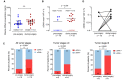
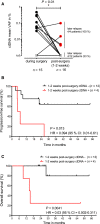
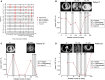
Circulating tumor DNA (ctDNA) has demonstrated great potential as a noninvasive biomarker to assess minimal residual disease (MRD) and profile tumor genotypes in patients with non-small-cell lung cancer (NSCLC). However, little is known about its dynamics during and after tumor resection, or its potential for predicting clinical outcomes. Here, we applied a targeted-capture high-throughput sequencing approach to profile ctDNA at various disease milestones and assessed its predictive value in patients with early-stage and locally advanced NSCLC. We prospectively enrolled 33 consecutive patients with stage IA to IIIB NSCLC undergoing curative-intent tumor resection (median follow-up: 26.2 months). From 21 patients, we serially collected 96 plasma samples before surgery, during surgery, 1-2 weeks postsurgery, and during follow-up. Deep next-generation sequencing using unique molecular identifiers was performed to identify and quantify tumor-specific mutations in ctDNA. Twelve patients (57%) had detectable mutations in ctDNA before tumor resection. Both ctDNA detection rates and ctDNA concentrations were significantly higher in plasma obtained during surgery compared with presurgical specimens (57% versus 19% ctDNA detection rate, and 12.47 versus 6.64 ng·mL-1 , respectively). Four patients (19%) remained ctDNA-positive at 1-2 weeks after surgery, with all of them (100%) experiencing disease progression at later time points. In contrast, only 4 out of 12 ctDNA-negative patients (33%) after surgery experienced relapse during follow-up. Positive ctDNA in early postoperative plasma samples was associated with shorter progression-free survival (P = 0.013) and overall survival (P = 0.004). Our findings suggest that, in early-stage and locally advanced NSCLC, intraoperative plasma sampling results in high ctDNA detection rates and that ctDNA positivity early after resection identifies patients at risk for relapse.
Keywords: circulating tumor DNA; early-stage and locally advanced non-small-cell lung cancer; minimal residual disease; noninvasive biomarker; relapse prediction.
© 2021 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
F.S. received research funding from Roche Sequencing Solutions. S.L. received Advisory Board and/or scientific meeting/presentation sponsorship; Agilent, AstraZeneca, Illumina, Novartis, Roche, as well as research project sponsorship Bristol–Myers–Squibb. All other authors declare no conflict of interest.
Figures
References
-
- Siegel RL, Miller KD & Jemal A (2020) Cancer statistics. CA Cancer J Clin 70, 7–30. - PubMed
-
- Detterbeck FC, Boffa DJ & Tanoue LT (2009) The new lung cancer staging system. Chest 136, 260–271. - PubMed
-
- Tanaka F & Yoneda K (2016) Adjuvant therapy following surgery in non‐small cell lung cancer (NSCLC). Surg Today 46, 25–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

