Making Nitronaphthalene Fluoresce
- PMID: 34653339
- PMCID: PMC8800371
- DOI: 10.1021/acs.jpclett.1c02155
Making Nitronaphthalene Fluoresce
Abstract
Nitroaromatic compounds are inherently nonfluorescent, and the subpicosecond lifetimes of the singlet excited states of many small nitrated polycyclic aromatic hydrocarbons, such as nitronaphthalenes, render them unfeasible for photosensitizers and photo-oxidants, despite their immensely beneficial reduction potentials. This article reports up to a 7000-fold increase in the singlet-excited-state lifetime of 1-nitronaphthalene upon attaching an amine or an N-amide to the ring lacking the nitro group. Varying the charge-transfer (CT) character of the excited states and the medium polarity balances the decay rates along the radiative and the two nonradiative pathways and can make these nitronaphthalene derivatives fluoresce. The strong electron-donating amine suppresses intersystem crossing (ISC) but accommodates CT pathways of nonradiate deactivation. Conversely, the N-amide does not induce a pronounced CT character but slows down ISC enough to achieve relatively long lifetimes of the singlet excited state. These paradigms are key for the pursuit of electron-deficient (n-type) organic conjugates with promising optical characteristics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

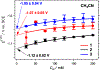
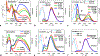


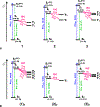
References
-
- Stolar M; Baumgartner T Organic n-type materials for charge transport and charge storage applications. Phys. Chem. Chem. Phys 2013, 15 (23), 9007–9024. - PubMed
-
- Yue Q; Liu W; Zhu X n-Type Molecular Photovoltaic Materials: Design Strategies and Device Applications. J. Am. Chem. Soc 2020, 142 (27), 11613–11628. - PubMed
-
- Wilson TM; Tauber MJ; Wasielewski MR Toward an n-Type Molecular Wire: Electron Hopping within Linearly Linked Perylenediimide Oligomers. J. Am. Chem. Soc 2009, 131 (25), 8952–8957. - PubMed
-
- Schuster DI; Cheng P; Jarowski PD; Guldi DM; Luo C; Echegoyen L; Pyo S; Holzwarth AR; Braslavsky SE; Williams RM; et al. Design, Synthesis, and Photophysical Studies of a Porphyrin-Fullerene Dyad with Parachute Topology; Charge Recombination in the Marcus Inverted Region. J. Am. Chem. Soc 2004, 126 (23), 7257–7270. - PubMed
-
- Lu H; Bao D; Penchev M; Ghazinejad M; Vullev VI; Ozkan CS; Ozkan M Pyridine-coated lead sulfide quantum dots for polymer hybrid photovoltaic devices. Adv. Sci. Lett 2010, 3 (2), 101–109.
Grants and funding
LinkOut - more resources
Full Text Sources

