Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system
- PMID: 34653510
- PMCID: PMC12050987
- DOI: 10.1016/j.expneurol.2021.113895
Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system
Abstract
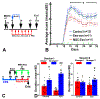
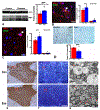
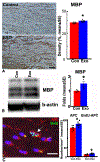
Injury of oligodendrocytes (OLs) induces demyelination, and patients with neurodegenerative diseases exhibit demyelination concomitantly with neurological deficit and cognitive impairment. Oligodendrocyte progenitor cells (OPCs) are present in the adult central nervous system (CNS), and they can proliferate, differentiate, and remyelinate axons after damage. However, remyelination therapies are not in clinical use. Multiple sclerosis (MS) is a major demyelinating disease in the CNS. Mesenchymal stromal cells (MSCs) have demonstrated therapeutic promise in animal models and in clinical trials of MS. Exosomes are nanoparticles generated by nearly all cells and they mediate cell-cell communication by transferring cargo biomaterials. Here, we hypothesize that exosomes harvested from MSCs have a similar therapeutic effect on enhancement of remyelination as that of MSCs. In the present study we employed exosomes derived from rhesus monkey MSCs (MSC-Exo). Two mouse models of demyelination were employed: 1) experimental autoimmune encephalomyelitis (EAE), an animal model of MS; and 2) cuprizone (CPZ) diet model, a toxic demyelination model. MSC-Exo or PBS were intravenously injected twice a week for 4 weeks, starting on day 10 post immunization in EAE mice, or once a week for 2 weeks starting on the day of CPZ diet withdrawal. Neurological and cognitive function were tested, OPC differentiation and remyelination, neuroinflammation and the potential underlying mechanisms were investigated using immunofluorescent staining, transmission electron microscopy and Western blot. Data generated from the EAE model revealed that MSC-Exo cross the blood brain barrier (BBB) and target neural cells. Compared with the controls (p < 0.05), treatment with MSC-Exo: 1) significantly improved neurological outcome; 2) significantly increased the numbers of newly generated OLs (BrdU+/APC+) and mature OLs (APC+), and the level of myelin basic protein (MBP); 3) decreased amyloid-β precursor protein (APP)+ density; 4) decreased neuroinflammation by increasing the M2 phenotype and decreasing the M1 phenotype of microglia, as well as their related cytokines; 5) inhibited the TLR2/IRAK1/NFκB pathway. Furthermore, we confirmed that the MSC-Exo treatment significantly improved cognitive function, promoted remyelination, increased polarization of M2 phenotype and blocked TLR2 signaling in the CPZ model. Collectively, MSC-Exo treatment promotes remyelination by both directly acting on OPCs and indirectly by acting on microglia in the demyelinating CNS. This study provides the cellular and molecular basis for this cell-free exosome therapy on remyelination and modulation of neuroinflammation in the CNS, with great potential for treatment of demyelinating and neurodegenerative disorders.
Keywords: Exosome; Mesenchymal stem cell; Microglia; Neuroinflammation; Oligodendrocyte; Oligodendrocyte progenitor cells; Remyelination.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
None.
Figures


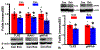

References
-
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ, 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol 29, 341–345. - PubMed
-
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF Jr., Rao MS, Sherman LS, 2005. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med 11, 966–972. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

