Distinct tumor-infiltrating lymphocyte landscapes are associated with clinical outcomes in localized non-small-cell lung cancer
- PMID: 34653632
- PMCID: PMC10019222
- DOI: 10.1016/j.annonc.2021.09.021
Distinct tumor-infiltrating lymphocyte landscapes are associated with clinical outcomes in localized non-small-cell lung cancer
Abstract
Background: Despite the importance of tumor-infiltrating T lymphocytes (TILs) in cancer biology, the relationship between TIL phenotypes and their prognostic relevance for localized non-small-cell lung cancer (NSCLC) has not been well established.
Patients and methods: Fresh tumor and normal adjacent tissue was prospectively collected from 150 patients with localized NSCLC. Tissue was comprehensively characterized by high-dimensional flow cytometry of TILs integrated with immunogenomic data from multiplex immunofluorescence, T-cell receptor sequencing, exome sequencing, RNA sequencing, targeted proteomics, and clinicopathologic features.
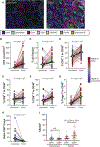
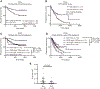
Results: While neither the magnitude of TIL infiltration nor specific TIL subsets were significantly prognostic alone, the integration of high-dimensional flow cytometry data identified two major immunotypes (IM1 and IM2) that were predictive of recurrence-free survival independent of clinical characteristics. IM2 was associated with poor prognosis and characterized by the presence of proliferating TILs expressing cluster of differentiation 103, programmed cell death protein 1, T-cell immunoglobulin and mucin-domain containing protein 3, and inducible T-cell costimulator. Conversely, IM1 was associated with good prognosis and differentiated by an abundance of CD8+ T cells expressing cytolytic enzymes, CD4+ T cells lacking the expression of inhibitory receptors, and increased levels of B-cell infiltrates and tertiary lymphoid structures. While increased B-cell infiltration was associated with good prognosis, the best prognosis was observed in patients with tumors exhibiting high levels of both B cells and T cells. These findings were validated in patient tumors from The Cancer Genome Atlas.
Conclusions: Our study suggests that although the number of infiltrating T cells is not associated with patient survival, the nature of the infiltrating T cells, resolved in distinct TIL immunotypes, is prognostically relevant in NSCLC and may inform therapeutic approaches to clinical care.
Keywords: T cells; biomarkers; immune system; lung cancer; microenvironment.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Disclosure CB has received research funding from Iovance Biotherapeutics and has participated in advisory committees for Myst Therapeutics and Turnstone Biologics. DLG has received research funding from AstraZeneca, Astellas, Janssen, Ribon Therapeutics, Takeda, and NGM Biopharmaceuticals, reports advisory role/consulting fees from AstraZeneca, Sanofi, Menarini Ricerche, and Eli Lilly, and research funding to MD Anderson Cancer Center from Boehringer Ingelheim. CH served on the advisory board for Briacell. SS has participated in advisory committees for Ethicon and for the Peter MacCallum Cancer Center. JVH has received research support from AstraZeneca, Bayer, GlaxoSmithKline, and Spectrum; participated in advisory committees for AstraZeneca, Boehringer Ingelheim, Exelixis, Genentech, GlaxoSmithKline, Guardant Health, Hengrui, Lilly, Novartis, Specrtum, EMD Serono, and Synta; and received royalties and/or licensing fees from Spectrum. TC has received speaker fees/honoraria from The Society for Immunotherapy of Cancer, Bristol Myers Squibb, Roche and Medscape Oncology, reports advisory role/consulting fees from MedImmune, AstraZeneca, Bristol Myers Squibb, EMD Serono, Merck & Co., Genentech, and Arrowhead Pharmaceuticals, and research funding to MD Anderson Cancer Center from Boehringer Ingelheim, MedImmune, AstraZeneca, Bristol Myers Squibb, and EMD Serono. JZ served on advisory board for AstraZeneca and Geneplus and received speaker’s fees from BMS, Geneplus, OrigMed, Innovent, grants from Merck, Johnson and Johnson from outside the submitted work. All other authors have declared no conflicts of interest.
Figures






Comment in
-
Insights from prospective multi-omic profiling of lymphocytes in resected lung cancer.Ann Oncol. 2022 Jan;33(1):4-5. doi: 10.1016/j.annonc.2021.10.013. Epub 2021 Oct 27. Ann Oncol. 2022. PMID: 34715315 No abstract available.
References
-
- Langer CJ, Besse B, Gualberto A, Brambilla E. & Soria JC The evolving role of histology in the management of advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28, 5311–5320, doi:10.1200/jco.2010.28.8126 (2010). - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

