Modulating endothelial cells with EGFL7 to diminish aGVHD after allogeneic bone marrow transplantation in mice
- PMID: 34654057
- PMCID: PMC9006300
- DOI: 10.1182/bloodadvances.2021005498
Modulating endothelial cells with EGFL7 to diminish aGVHD after allogeneic bone marrow transplantation in mice
Abstract
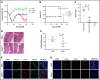
Acute graft-versus-host disease (aGVHD) is the second most common cause of death after allogeneic hematopoietic stem cell transplantation (allo-HSCT), underscoring the need for novel therapies. Based on previous work that endothelial cell dysfunction is present in aGVHD and that epidermal growth factor-like domain 7 (EGFL7) plays a significant role in decreasing inflammation by repressing endothelial cell activation and T-cell migration, we hypothesized that increasing EGFL7 levels after allo-HSCT will diminish the severity of aGVHD. Here, we show that treatment with recombinant EGFL7 (rEGFL7) in 2 different murine models of aGVHD decreases aGVHD severity and improves survival in recipient mice after allogeneic transplantation with respect to controls without affecting graft-versus-leukemia effect. Furthermore, we showed that rEGFL7 treatment results in higher thymocytes, T, B, and dendritic cell counts in recipient mice after allo-HSCT. This study constitutes a proof of concept of the ability of rEGFL7 therapy to reduce GHVD severity and mortality after allo-HSCT.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

References
-
- Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324(10):667-674. - PubMed
-
- Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8(6):575-581. - PubMed
-
- Matte CC, Liu J, Cormier J, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10(9):987-992. - PubMed

