Functional role of brain-engrafted macrophages against brain injuries
- PMID: 34654458
- PMCID: PMC8520231
- DOI: 10.1186/s12974-021-02290-0
Functional role of brain-engrafted macrophages against brain injuries
Abstract
Background: Brain-resident microglia have a distinct origin compared to macrophages in other organs. Under physiological conditions, microglia are maintained by self-renewal from the local pool, independent of hematopoietic progenitors. Pharmacological depletion of microglia during whole-brain radiotherapy prevents synaptic loss and long-term recognition memory deficits. However, the origin or repopulated cells and the mechanisms behind these protective effects are unknown.
Methods: CD45low/int/CD11b+ cells from naïve brains, irradiated brains, PLX5622-treated brains and PLX5622 + whole-brain radiotherapy-treated brains were FACS sorted and sequenced for transcriptomic comparisons. Bone marrow chimeras were used to trace the origin and long-term morphology of repopulated cells after PLX5622 and whole-brain radiotherapy. FACS analyses of intrinsic and exotic synaptic compartments were used to measure phagocytic activities of microglia and repopulated cells. In addition, concussive brain injuries were given to PLX5622 and brain-irradiated mice to study the potential protective functions of repopulated cells after PLX5622 + whole-brain radiotherapy.
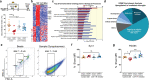
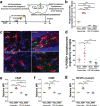
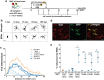
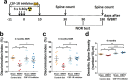
Results: After a combination of whole-brain radiotherapy and microglia depletion, repopulated cells are brain-engrafted macrophages that originate from circulating monocytes. Comparisons of transcriptomes reveal that brain-engrafted macrophages have an intermediate phenotype that resembles both monocytes and embryonic microglia. In addition, brain-engrafted macrophages display reduced phagocytic activity for synaptic compartments compared to microglia from normal brains in response to a secondary concussive brain injury. Importantly, replacement of microglia by brain-engrafted macrophages spare mice from whole-brain radiotherapy-induced long-term cognitive deficits, and prevent concussive injury-induced memory loss.
Conclusions: Brain-engrafted macrophages prevent radiation- and concussion-induced brain injuries and cognitive deficits.
Keywords: Brain irradiation; Brain-engrafted macrophages; CSF-1R inhibitor; Cognition; Microglia.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

