Biogeographic traits of dimethyl sulfide and dimethylsulfoniopropionate cycling in polar oceans
- PMID: 34654476
- PMCID: PMC8520302
- DOI: 10.1186/s40168-021-01153-3
Biogeographic traits of dimethyl sulfide and dimethylsulfoniopropionate cycling in polar oceans
Erratum in
-
Correction to: Biogeographic traits of dimethyl sulfide and dimethylsulfoniopropionate cycling in polar oceans.Microbiome. 2021 Nov 9;9(1):221. doi: 10.1186/s40168-021-01182-y. Microbiome. 2021. PMID: 34753519 Free PMC article. No abstract available.
Abstract
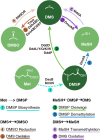
Background: Dimethyl sulfide (DMS) is the dominant volatile organic sulfur in global oceans. The predominant source of oceanic DMS is the cleavage of dimethylsulfoniopropionate (DMSP), which can be produced by marine bacteria and phytoplankton. Polar oceans, which represent about one fifth of Earth's surface, contribute significantly to the global oceanic DMS sea-air flux. However, a global overview of DMS and DMSP cycling in polar oceans is still lacking and the key genes and the microbial assemblages involved in DMSP/DMS transformation remain to be fully unveiled.
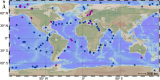
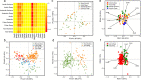
Results: Here, we systematically investigated the biogeographic traits of 16 key microbial enzymes involved in DMS/DMSP cycling in 60 metagenomic samples from polar waters, together with 174 metagenome and 151 metatranscriptomes from non-polar Tara Ocean dataset. Our analyses suggest that intense DMS/DMSP cycling occurs in the polar oceans. DMSP demethylase (DmdA), DMSP lyases (DddD, DddP, and DddK), and trimethylamine monooxygenase (Tmm, which oxidizes DMS to dimethylsulfoxide) were the most prevalent bacterial genes involved in global DMS/DMSP cycling. Alphaproteobacteria (Pelagibacterales) and Gammaproteobacteria appear to play prominent roles in DMS/DMSP cycling in polar oceans. The phenomenon that multiple DMS/DMSP cycling genes co-occurred in the same bacterial genome was also observed in metagenome assembled genomes (MAGs) from polar oceans. The microbial assemblages from the polar oceans were significantly correlated with water depth rather than geographic distance, suggesting the differences of habitats between surface and deep waters rather than dispersal limitation are the key factors shaping microbial assemblages involved in DMS/DMSP cycling in polar oceans.
Conclusions: Overall, this study provides a global overview of the biogeographic traits of known bacterial genes involved in DMS/DMSP cycling from the Arctic and Antarctic oceans, laying a solid foundation for further studies of DMS/DMSP cycling in polar ocean microbiome at the enzymatic, metabolic, and processual levels. Video Abstract.
Keywords: DMS/DMSP cycling; Geographic distribution; Phylogenetic diversity; Polar oceans.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Gondwe M, Krol M, Gieskes W, Klaassen W, de Baar H. The contribution of ocean-leaving DMS to the global atmospheric burdens of DMS, MSA, SO2, and NSS SO4= Glob Biogeochem Cycles. 2003;17:25.
-
- Mahajan AS, Fadnavis S, Thomas MA, Pozzoli L, Gupta S, Royer S-J, Saiz-Lopez A, Simó R. Quantifying the impacts of an updated global dimethyl sulfide climatology on cloud microphysics and aerosol radiative forcing. J Geophys Res Atmos. 2015;120:2524–2536. doi: 10.1002/2014JD022687. - DOI
-
- Spiese CE, Kieber DJ, Nomura CT, Kiene RP. Reduction of dimethylsulfoxide to dimethylsulfide by marine phytoplankton. Limnol Oceanogr. 2009;54:560–570. doi: 10.4319/lo.2009.54.2.0560. - DOI
-
- Deschaseaux E, Jones GB, Swan HB. Dimethylated sulfur compounds in coral-reef ecosystems. Environ Chem. 2016;13:239–251. doi: 10.1071/EN14258. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

