The peroxidatic activities of Myoglobin and Hemoglobin, their pathological consequences and possible medical interventions
- PMID: 34654576
- PMCID: PMC8837633
- DOI: 10.1016/j.mam.2021.101045
The peroxidatic activities of Myoglobin and Hemoglobin, their pathological consequences and possible medical interventions
Abstract
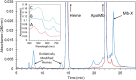
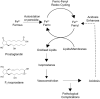
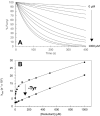
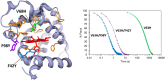
Under those pathological conditions in which Myoglobin and Hemoglobin escape their cellular environments and are thus separated from cellular reductive/protective systems, the inherent peroxidase activities of these proteins can be expressed. This activity leads to the formation of the highly oxidizing oxo-ferryl species. Evidence that this happens in vivo is provided by the formation of a covalent bond between the heme group and the protein and this acts as an unambiguous biomarker for the presence of the oxo ferryl form. The peroxidatic activity also leads to the oxidation of lipids, the products of which can be powerful vasoconstrictive agents (e.g. isoprostanes, neuroprostanes). Here we review the evidence that lipid oxidation occurs following rhabdomyolysis and sub-arachnoid hemorrhage and that the products formed from arachidonic acid chains of phospholipids lead, through vasoconstriction, to kidney failure and brain vasospasm. Intervention in these pathological conditions through administration of reducing agents to remove ferryl heme is discussed. Through-protein electron transfer pathways that facilitate ferryl reduction at low reductant concentration have been identified. We conclude with consideration of the therapeutic use of Hemoglobin Based Oxygen carriers and how the toxicity of these may be reduced by engineering such electron transfer pathways into hemoglobin.
Keywords: Blood substitute; Ferryl; Hemoglobin; Myoglobin; Peroxidase; Rhabdomyolysis.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Abuchowski A. SANGUINATE (PEGylated carboxyhemoglobin bovine): mechanism of action and clinical update. Artif. Organs. 2017;41(4):346–350. - PubMed
-
- Alayash A.I. betaCysteine 93 in human hemoglobin: a gateway to oxidative stability in health and disease. Lab. Invest. 2021;101(1):4–11. - PubMed
-
- Anderson B.J. Paracetamol (Acetaminophen): mechanisms of action. Paediatr. Anaesth. 2008;18(10):915–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- B18662/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/T025441/1/MRC_/Medical Research Council/United Kingdom
- BB/F007663/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/L01310X/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources

