Muscular dystrophy-dystroglycanopathy in a family of Labrador retrievers with a LARGE1 mutation
- PMID: 34654610
- PMCID: PMC8963908
- DOI: 10.1016/j.nmd.2021.07.016
Muscular dystrophy-dystroglycanopathy in a family of Labrador retrievers with a LARGE1 mutation
Abstract
Alpha-dystroglycan (αDG) is a highly glycosylated cell surface protein with a significant role in cell-to-extracellular matrix interactions in muscle. αDG interaction with extracellular ligands relies on the activity of the LARGE1 glycosyltransferase that synthesizes and extends the heteropolysaccharide matriglycan. Abnormalities in αDG glycosylation and formation of matriglycan are the pathogenic mechanisms for the dystroglycanopathies, a group of congenital muscular dystrophies. Muscle biopsies were evaluated from related 6-week-old Labrador retriever puppies with poor suckling, small stature compared to normal litter mates, bow-legged stance and markedly elevated creatine kinase activities. A dystrophic phenotype with marked degeneration and regeneration, multifocal mononuclear cell infiltration and endomysial fibrosis was identified on muscle cryosections. Single nucleotide polymorphism (SNP) array genotyping data on the family members identified three regions of homozygosity in 4 cases relative to 8 controls. Analysis of whole genome sequence data from one of the cases identified a stop codon mutation in the LARGE1 gene that truncates 40% of the protein. Immunofluorescent staining and western blotting demonstrated the absence of matriglycan in skeletal muscle and heart from affected dogs. Compared to control, LARGE enzyme activity was not detected. This is the first report of a dystroglycanopathy in dogs.
Keywords: Dog; Glycosylation; Myopathy; α-dystroglycan.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






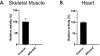
References
-
- Longman C, Brockington M, Torelli S, Jimenez-Mallebrera C, Kennedy C, Khalil N et al. Mutations in the human LARGE gene cause CMD1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet 2003;12:2853–61. doi: 10.1093/hmg/ddg307. - DOI - PubMed

