Targeting LRRC15 Inhibits Metastatic Dissemination of Ovarian Cancer
- PMID: 34654724
- PMCID: PMC8930558
- DOI: 10.1158/0008-5472.CAN-21-0622
Targeting LRRC15 Inhibits Metastatic Dissemination of Ovarian Cancer
Abstract
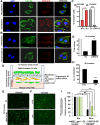
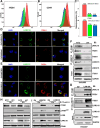
Dissemination of ovarian cancer cells can lead to inoperable metastatic lesions in the bowel and omentum that cause patient death. Here we show that LRRC15, a type-I 15-leucine-rich repeat-containing membrane protein, highly overexpressed in ovarian cancer bowel metastases compared with matched primary tumors and acts as a potent promoter of omental metastasis. Complementary models of ovarian cancer demonstrated that LRRC15 expression leads to inhibition of anoikis-induced cell death and promotes adhesion and invasion through matrices that mimic omentum. Mechanistically, LRRC15 interacted with β1-integrin to stimulate activation of focal adhesion kinase (FAK) signaling. As a therapeutic proof of concept, targeting LRRC15 with the specific antibody-drug conjugate ABBV-085 in both early and late metastatic ovarian cancer cell line xenograft models prevented metastatic dissemination, and these results were corroborated in metastatic patient-derived ovarian cancer xenograft models. Furthermore, treatment of 3D-spheroid cultures of LRRC15-positive patient-derived ascites with ABBV-085 reduced cell viability. Overall, these data uncover a role for LRRC15 in promoting ovarian cancer metastasis and suggest a novel and promising therapy to target ovarian cancer metastases.
Significance: This study identifies that LRRC15 activates β1-integrin/FAK signaling to promote ovarian cancer metastasis and shows that the LRRC15-targeted antibody-drug conjugate ABBV-085 suppresses ovarian cancer metastasis in preclinical models.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures







References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. - PubMed
-
- Mangili G, Aletti G, Frigerio L, Franchi M, Panacci N, Viganò R, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 2005;15:830–5. - PubMed
-
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000:Cd002764.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

