Human induced pluripotent stem cell-derived platelets loaded with lapatinib effectively target HER2+ breast cancer metastasis to the brain
- PMID: 34654856
- PMCID: PMC8521584
- DOI: 10.1038/s41598-021-96351-2
Human induced pluripotent stem cell-derived platelets loaded with lapatinib effectively target HER2+ breast cancer metastasis to the brain
Retraction in
-
Retraction Note: Human induced pluripotent stem cell-derived platelets loaded with lapatinib effectively target HER2+ breast cancer metastasis to the brain.Sci Rep. 2024 Mar 12;14(1):5972. doi: 10.1038/s41598-024-56291-z. Sci Rep. 2024. PMID: 38472273 Free PMC article. No abstract available.
Abstract
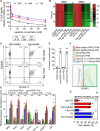
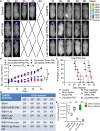
Prognosis of patients with HER2+ breast-to-brain-metastasis (BBM) is dismal even after current standard-of-care treatments, including surgical resection, whole-brain radiation, and systemic chemotherapy. Radiation and systemic chemotherapies can also induce cytotoxicity, leading to significant side effects. Studies indicate that donor-derived platelets can serve as immune-compatible drug carriers that interact with and deliver drugs to cancer cells with fewer side effects, making them a promising therapeutic option with enhanced antitumor activity. Moreover, human induced pluripotent stem cells (hiPSCs) provide a potentially renewable source of clinical-grade transfusable platelets that can be drug-loaded to complement the supply of donor-derived platelets. Here, we describe methods for ex vivo generation of megakaryocytes (MKs) and functional platelets from hiPSCs (hiPSC-platelets) in a scalable fashion. We then loaded hiPSC-platelets with lapatinib and infused them into BBM tumor-bearing NOD/SCID mouse models. Such treatment significantly increased intracellular lapatinib accumulation in BBMs in vivo, potentially via tumor cell-induced activation/aggregation. Lapatinib-loaded hiPSC-platelets exhibited normal morphology and function and released lapatinib pH-dependently. Importantly, lapatinib delivery to BBM cells via hiPSC-platelets inhibited tumor growth and prolonged survival of tumor-bearing mice. Overall, use of lapatinib-loaded hiPSC-platelets effectively reduced adverse effects of free lapatinib and enhanced its therapeutic efficacy, suggesting that they represent a novel means to deliver chemotherapeutic drugs as treatment for BBM.
© 2021. The Author(s).
Conflict of interest statement
Rahul Jandial is a principal investigator on a grant to City of Hope National Medical Center from Department of Defense. City of Hope National medical center has pending patent application related to findings in this manuscript. The authors Arunoday Bhan, and Rahul Jandial are named as inventors in these applications.
Figures



Comment in
-
Findings of Research Misconduct.Fed Regist. 2024 Sep 17;89(180):76121-76123. Fed Regist. 2024. PMID: 39360268 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

