Small molecule compound M12 reduces vascular permeability in obese mice via blocking endothelial TRPV4-Nox2 interaction
- PMID: 34654876
- PMCID: PMC9160247
- DOI: 10.1038/s41401-021-00780-8
Small molecule compound M12 reduces vascular permeability in obese mice via blocking endothelial TRPV4-Nox2 interaction
Abstract
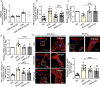
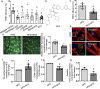
Transient receptor potential channel TRPV4 and nicotinamide adenine dinucleotide phosphate oxidase (Nox2) are involved in oxidative stress that increases endothelial permeability. It has been shown that obesity enhances the physical association of TRPV4 and Nox2, but the role of TRPV4-Nox2 association in obesity has not been clarified. In this study we investigated the function of TRPV4-Nox2 complex in reducing oxidative stress and regulating abnormal vascular permeability in obesity. Obesity was induced in mice by feeding a high-fat diet (HFD) for 14 weeks. The physical interaction between TRPV4 and Nox2 was measured using FRET, co-immunoprecipitation and GST pull-down assays. The functional interaction was measured by rhodamine phalloidin, CM-H2DCFDA in vitro, the fluorescent dye dihydroethidium (DHE) staining assay, and the Evans blue permeability assay in vivo. We demonstrated that TRPV4 physically and functionally associated with Nox2, and this physical association was enhanced in aorta of obese mice. Furthermore, we showed that interrupting TRPV4-Nox2 coupling by TRPV4 knockout, or by treatment with a specific Nox2 inhibitor Nox2 dstat or a specific TRPV4 inhibitor HC067046 significantly attenuated obesity-induced ROS overproduction in aortic endothelial cells, and reversed the abnormal endothelial cytoskeletal structure. In order to discover small molecules disrupting the over-coupling of TPRV4 and Nox2 in obesity, we performed molecular docking analysis and found that compound M12 modulated TRPV4-Nox2 association, reduced ROS production, and finally reversed disruption of the vascular barrier in obesity. Together, this study, for the first time, provides evidence for the TRPV4 physically interacting with Nox2. TRPV4-Nox2 complex is a potential drug target in improving oxidative stress and disruption of the vascular barrier in obesity. Compound M12 targeting TRPV4-Nox2 complex can improve vascular barrier function in obesity.
Keywords: Compound M12; Nox2; TPRV4; obesity; oxidant stress; vascular permeability.
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
Conflict of interest statement
XM, CLT, MRG, and JH have applied to the State Intellectual Property Office of China for the patent “A compound for reducing TRPV4–Nox2 excessive interaction and its application in anti-abnormal vascular permeability” (2020114600923).
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

