Liprins in oncogenic signaling and cancer cell adhesion
- PMID: 34654889
- PMCID: PMC8602034
- DOI: 10.1038/s41388-021-02048-1
Liprins in oncogenic signaling and cancer cell adhesion
Abstract
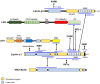
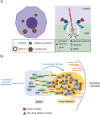
Liprins are a multifunctional family of scaffold proteins, identified by their involvement in several important neuronal functions related to signaling and organization of synaptic structures. More recently, the knowledge on the liprin family has expanded from neuronal functions to processes relevant to cancer progression, including cell adhesion, cell motility, cancer cell invasion, and signaling. These proteins consist of regions, which by prediction are intrinsically disordered, and may be involved in the assembly of supramolecular structures relevant for their functions. This review summarizes the current understanding of the functions of liprins in different cellular processes, with special emphasis on liprins in tumor progression. The available data indicate that liprins may be potential biomarkers for cancer progression and may have therapeutic importance.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Serra-Pages C, Medley QG, Tang M, Hart A, Streuli M. Liprins, a family of LAR transmembrane protein-tyrosine phosphatase-interacting proteins. J Biol Chem. 1998;273:15611–20. - PubMed
-
- de Curtis I. Function of liprins in cell motility. Exp Cell Res. 2011;317:1–8. - PubMed
-
- Chiaretti S, de Curtis I. Role of Liprins in the Regulation of Tumor Cell Motility and Invasion. Curr Cancer Drug Targets. 2016;16:238–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

