RIPK3 signaling and its role in the pathogenesis of cancers
- PMID: 34654937
- PMCID: PMC9044760
- DOI: 10.1007/s00018-021-03947-y
RIPK3 signaling and its role in the pathogenesis of cancers
Abstract
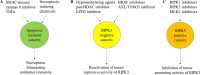
RIPK3 (receptor-interacting protein kinase 3) is a serine/threonine-protein kinase. As a key component of necrosomes, RIPK3 is an essential mediator of inflammatory factors (such as TNFα-tumor necrosis factor α) and infection-induced necroptosis, a programmed necrosis. In addition, RIPK3 signaling is also involved in the regulation of apoptosis, cytokine/chemokine production, mitochondrial metabolism, autophagy, and cell proliferation by interacting with and/or phosphorylating the critical regulators of the corresponding signaling pathways. Similar to apoptosis, RIPK3-signaling-mediated necroptosis is inactivated in most types of cancers, suggesting RIPK3 might play a critical suppressive role in the pathogenesis of cancers. However, in some inflammatory types of cancers, such as pancreatic cancers and colorectal cancers, RIPK3 signaling might promote cancer development by stimulating proliferation signaling in tumor cells and inducing an immunosuppressive response in the tumor environment. In this review, we summarize recent research progress in the regulators of RIPK3 signaling, and discuss the function of this pathway in the regulation of mixed lineage kinase domain-like (MLKL)-mediated necroptosis and MLKL-independent cellular behaviors. In addition, we deliberate the potential roles of RIPK3 signaling in the pathogenesis of different types of cancers and discuss the potential strategies for targeting this pathway in cancer therapy.
Keywords: Cancer pathogenesis; MLKL necroptosis; MLKL-independent signaling; RIPK3 signaling.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no competing financial or professional interests.
Figures




References
-
- Daniels BP, Kofman SB, Smith JR, Norris GT, Snyder AG, Kolb JP, Gao X, Locasale JW, Martinez J, Gale M, Jr, et al. The nucleotide sensor ZBP1 and kinase RIPK3 induce the enzyme IRG1 to promote an antiviral metabolic state in neurons. Immunity. 2019;50(1):64–76e64. doi: 10.1016/j.immuni.2018.11.017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

