Edited magnetic resonance spectroscopy in the neonatal brain
- PMID: 34654960
- PMCID: PMC8887832
- DOI: 10.1007/s00234-021-02821-9
Edited magnetic resonance spectroscopy in the neonatal brain
Abstract
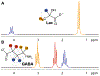
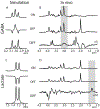
J-difference-edited spectroscopy is a valuable approach for the detection of low-concentration metabolites with magnetic resonance spectroscopy (MRS). Currently, few edited MRS studies are performed in neonates due to suboptimal signal-to-noise ratio, relatively long acquisition times, and vulnerability to motion artifacts. Nonetheless, the technique presents an exciting opportunity in pediatric imaging research to study rapid maturational changes of neurotransmitter systems and other metabolic systems in early postnatal life. Studying these metabolic processes is vital to understanding the widespread and rapid structural and functional changes that occur in the first years of life. The overarching goal of this review is to provide an introduction to edited MRS for neonates, including the current state-of-the-art in editing methods and editable metabolites, as well as to review the current literature applying edited MRS to the neonatal brain. Existing challenges and future opportunities, including the lack of age-specific reference data, are also discussed.
Keywords: Edited MRS; J-difference editing; Low-concentration metabolites; Neonatal brain; Relaxation time.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures
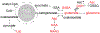


References
-
- Pouwels PJ et al. (1999) Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr Res 46:474–485 - PubMed
-
- Kreis R et al. (2002) Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 48:949–958 - PubMed
-
- Durston S et al. (2001) Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry 40:1012–1020 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 EB031771/EB/NIBIB NIH HHS/United States
- R00 AG062230/AG/NIA NIH HHS/United States
- K23 HD099309/HD/NICHD NIH HHS/United States
- 1R34DA050292-01/DA/NIDA NIH HHS/United States
- P41 EB 031771/NH/NIH HHS/United States
- P50 HD103538/HD/NICHD NIH HHS/United States
- R01 MH106564/MH/NIMH NIH HHS/United States
- R21 HD100869/HD/NICHD NIH HHS/United States
- R01 EB 023963/NH/NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- GN 2728/action medical research
- P41 EB015909/EB/NIBIB NIH HHS/United States
- R21 MH098228/MH/NIMH NIH HHS/United States
- R01 EB016089/EB/NIBIB NIH HHS/United States
- P50 HD105351/HD/NICHD NIH HHS/United States
- R01 EB023963/EB/NIBIB NIH HHS/United States
- MR/P008712/1/MRC_/Medical Research Council/United Kingdom
- R21 HD 100869/NH/NIH HHS/United States
- WT 203148/Z/16/Z/wellcome engineering and physical sciences research countil
- MR/V 036874/1/MRC_/Medical Research Council/United Kingdom
- MR/V036874/1/MRC_/Medical Research Council/United Kingdom
- K23 HD 099309/NH/NIH HHS/United States
- K99 AG062230/AG/NIA NIH HHS/United States
- R34 DA050292/DA/NIDA NIH HHS/United States
- R00 AG 062230/NH/NIH HHS/United States
- R01 EB016089/NH/NIH HHS/United States
- MR/N026063/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

