Long-Term Immune Recovery After Hematopoietic Stem Cell Transplantation for ADA Deficiency: a Single-Center Experience
- PMID: 34654999
- PMCID: PMC8821083
- DOI: 10.1007/s10875-021-01145-w
Long-Term Immune Recovery After Hematopoietic Stem Cell Transplantation for ADA Deficiency: a Single-Center Experience
Abstract
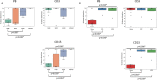
Unconditioned hematopoietic stem cell transplantation (HSCT) is the recommended treatment for patients with adenosine deaminase (ADA)-deficient severe combined immunodeficiency with an HLA-matched sibling donor (MSD) or family donor (MFD). Improved overall survival (OS) has been reported compared to the use of unrelated donors, and previous studies have demonstrated that adequate cellular and humoral immune recovery can be achieved even in the absence of conditioning. Detailed insight of the long-term outcome is still limited. We aim to address this by studying a large single-center cohort of 28 adenosine deaminase-deficient patients who underwent a total of 31 HSCT procedures, of which more than half were unconditioned. We report an OS of 85.7% and event-free survival of 71% for the entire cohort, with no statistically significant differences after procedures using related or unrelated HLA-matched donors. We find that donor engraftment in the myeloid compartment is significantly diminished in unconditioned procedures, which typically use a MSD or MFD. This is associated with poor metabolic correction and more frequent failure to discontinue immunoglobulin replacement therapy. Approximately one in four patients receiving an unconditioned procedure required a second procedure, whereas the use of reduced intensity conditioning (RIC) prior to allogeneic transplantation improves the long-term outcome by achieving better myeloid engraftment, humoral immune recovery, and metabolic correction. Further longitudinal studies are needed to optimize future management and guidelines, but our findings support a potential role for the routine use of RIC in most ADA-deficient patients receiving an HLA-identical hematopoietic stem cell transplant, even when a MSD or MFD is available.
Keywords: ADA SCID; Hematopoietic stem cell transplantation; Primary immunodeficiency; Reduced intensity conditioning.
© 2021. The Author(s).
Conflict of interest statement
CB has received educational honoraria from GSK and Orchard Therapeutics related to ADA-SCID. HBG is an employee of Orchard Therapeutics and holds equity in the company.
Figures



References
-
- Gaspar HB. Bone marrow transplantation and alternatives for adenosine deaminase deficiency. Immunol Allergy Clin North Am. 2010;30(2):221–236. - PubMed
-
- Bradford KL, et al. Adenosine deaminase (ADA)-deficient severe combined immune deficiency (SCID): molecular pathogenesis and clinical manifestations. J Clin Immunol. 2017;37(7):626–637. - PubMed
-
- Van der Weyden MB, Kelley WN. Human adenosine deaminase Distribution and properties. J Biol Chem. 1976;251(18):5448–5456. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

