BrainGNN: Interpretable Brain Graph Neural Network for fMRI Analysis
- PMID: 34655865
- PMCID: PMC9916535
- DOI: 10.1016/j.media.2021.102233
BrainGNN: Interpretable Brain Graph Neural Network for fMRI Analysis
Abstract
Understanding which brain regions are related to a specific neurological disorder or cognitive stimuli has been an important area of neuroimaging research. We propose BrainGNN, a graph neural network (GNN) framework to analyze functional magnetic resonance images (fMRI) and discover neurological biomarkers. Considering the special property of brain graphs, we design novel ROI-aware graph convolutional (Ra-GConv) layers that leverage the topological and functional information of fMRI. Motivated by the need for transparency in medical image analysis, our BrainGNN contains ROI-selection pooling layers (R-pool) that highlight salient ROIs (nodes in the graph), so that we can infer which ROIs are important for prediction. Furthermore, we propose regularization terms-unit loss, topK pooling (TPK) loss and group-level consistency (GLC) loss-on pooling results to encourage reasonable ROI-selection and provide flexibility to encourage either fully individual- or patterns that agree with group-level data. We apply the BrainGNN framework on two independent fMRI datasets: an Autism Spectrum Disorder (ASD) fMRI dataset and data from the Human Connectome Project (HCP) 900 Subject Release. We investigate different choices of the hyper-parameters and show that BrainGNN outperforms the alternative fMRI image analysis methods in terms of four different evaluation metrics. The obtained community clustering and salient ROI detection results show a high correspondence with the previous neuroimaging-derived evidence of biomarkers for ASD and specific task states decoded for HCP. Our code is available at https://github.com/xxlya/BrainGNN_Pytorch.
Keywords: ASD; Biomarker; GNN; fMRI.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





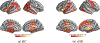




References
-
- Abraham A, Milham MP, Di Martino A, Craddock RC, Samaras D, Thirion B, Varoquaux G, 2017. Deriving reproducible biomarkers from multi-site resting-state data: an autism-based example. NeuroImage 147, 736–745. - PubMed
-
- Adebayo J, Gilmer J, Muelly M, Goodfellow I, Hardt M, Kim B, 2018. Sanity checks for saliency maps. Advances in Neural Information Processing Systems.
-
- Bai Y, Calhoun VD, Wang Y-P, 2020. Integration of multi-task fmri for cognitive study by structure-enforced collaborative regression. In: Medical Imaging 2020: Biomedical Applications in Molecular, Structural, and Functional Imaging, 11317. International Society for Optics and Photonics, p. 1131722.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

