Extracellular vesicles: mediators of intercellular communication in tissue injury and disease
- PMID: 34656117
- PMCID: PMC8520651
- DOI: 10.1186/s12964-021-00787-y
Extracellular vesicles: mediators of intercellular communication in tissue injury and disease
Abstract
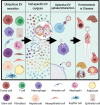
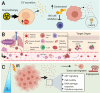
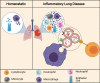
Intercellular communication is a critical process that ensures cooperation between distinct cell types and maintains homeostasis. EVs, which were initially described as cellular debris and devoid of biological function, are now recognized as key components in cell-cell communication. EVs are known to carry multiple factors derived from their cell of origin, including cytokines and chemokines, active enzymes, metabolites, nucleic acids, and surface molecules, that can alter the behavior of recipient cells. Since the cargo of EVs reflects their parental cells, EVs from damaged and dysfunctional tissue environments offer an abundance of information toward elucidating the molecular mechanisms of various diseases and pathological conditions. In this review, we discuss the most recent findings regarding the role of EVs in the progression of cancer, metabolic disorders, and inflammatory lung diseases given the high prevalence of these conditions worldwide and the important role that intercellular communication between immune, parenchymal, and stromal cells plays in the development of these pathological states. We also consider the clinical applications of EVs, including the possibilities for their use as novel therapeutics. While intercellular communication through extracellular vesicles (EVs) is key for physiological processes and tissue homeostasis, injury and stress result in altered communication patterns in the tissue microenvironment. When left unchecked, EV-mediated interactions between stromal, immune, and parenchymal cells lead to the development of disease states Video Abstract.
Keywords: Extracellular vesicles; Immune response; Intercellular communication; Lung inflammation; Metabolic disorders; Reprogramming; Tumor microenvironment.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

