Sodium phenylbutyrate inhibits Schwann cell inflammation via HDAC and NFκB to promote axonal regeneration and remyelination
- PMID: 34656124
- PMCID: PMC8520633
- DOI: 10.1186/s12974-021-02273-1
Sodium phenylbutyrate inhibits Schwann cell inflammation via HDAC and NFκB to promote axonal regeneration and remyelination
Abstract
Background: Epigenetic regulation by histone deacetylases (HDACs) in Schwann cells (SCs) after injury facilitates them to undergo de- and redifferentiation processes necessary to support various stages of nerve repair. Although de-differentiation activates the synthesis and secretion of inflammatory cytokines by SCs to initiate an immune response during nerve repair, changes in either the timing or duration of prolonged inflammation mediated by SCs can affect later processes associated with repair and regeneration. Limited studies have investigated the regulatory processes through which HDACs in SCs control inflammatory cytokines to provide a favorable environment for peripheral nerve regeneration.
Methods: We employed the HDAC inhibitor (HDACi) sodium phenylbutyrate (PBA) to address this question in an in vitro RT4 SC inflammation model and an in vivo sciatic nerve transection injury model to examine the effects of HDAC inhibition on the expression of pro-inflammatory cytokines. Furthermore, we assessed the outcomes of suppression of extended inflammation on the regenerative potential of nerves by assessing axonal regeneration, remyelination, and reinnervation.
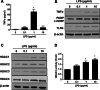
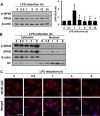
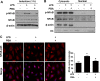
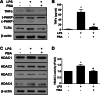
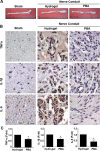
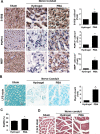
Results: Significant reductions in lipopolysaccharide (LPS)-induced pro-inflammatory cytokine (tumor necrosis factor-α [TNFα]) expression and secretion were observed in vitro following PBA treatment. PBA treatment also affected the transient changes in nuclear factor κB (NFκB)-p65 phosphorylation and translocation in response to LPS induction in RT4 SCs. Similarly, PBA mediated long-term suppressive effects on HDAC3 expression and activity. PBA administration resulted in marked inhibition of pro-inflammatory cytokine secretion at the site of transection injury when compared with that in the hydrogel control group at 6-week post-injury. A conducive microenvironment for axonal regrowth and remyelination was generated by increasing expression levels of protein gene product 9.5 (PGP9.5) and myelin basic protein (MBP) in regenerating nerve tissues. PBA administration increased the relative gastrocnemius muscle weight percentage and maintained the intactness of muscle bundles when compared with those in the hydrogel control group.
Conclusions: Suppressing the lengthened state of inflammation using PBA treatment favors axonal regrowth and remyelination following nerve transection injury. PBA treatment also regulates pro-inflammatory cytokine expression by inhibiting the transcriptional activation of NFκB-p65 and HDAC3 in SCs in vitro.
Keywords: HDAC inhibitor; Inflammation; Peripheral nerve injury; Regeneration and myelination; Schwann cells.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures
Similar articles
-
Emerging Role of HDACs in Regeneration and Ageing in the Peripheral Nervous System: Repair Schwann Cells as Pivotal Targets.Int J Mol Sci. 2022 Mar 10;23(6):2996. doi: 10.3390/ijms23062996. Int J Mol Sci. 2022. PMID: 35328416 Free PMC article. Review.
-
GDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in rats.J Tissue Eng Regen Med. 2018 Jan;12(1):e398-e407. doi: 10.1002/term.2431. Epub 2017 Jun 26. J Tissue Eng Regen Med. 2018. PMID: 28296347
-
Schwann cell O-GlcNAcylation promotes peripheral nerve remyelination via attenuation of the AP-1 transcription factor JUN.Proc Natl Acad Sci U S A. 2018 Jul 31;115(31):8019-8024. doi: 10.1073/pnas.1805538115. Epub 2018 Jul 16. Proc Natl Acad Sci U S A. 2018. PMID: 30012597 Free PMC article.
-
Nuclear factor-kappaB activation in axons and Schwann cells in experimental sciatic nerve injury and its role in modulating axon regeneration: studies with etanercept.J Neuropathol Exp Neurol. 2009 Jun;68(6):691-700. doi: 10.1097/NEN.0b013e3181a7c14e. J Neuropathol Exp Neurol. 2009. PMID: 19458540 Free PMC article.
-
Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury.J Neuroinflammation. 2011 Aug 30;8:110. doi: 10.1186/1742-2094-8-110. J Neuroinflammation. 2011. PMID: 21878126 Free PMC article. Review.
Cited by
-
Amyotrophic Lateral Sclerosis in Long-COVID Scenario and the Therapeutic Potential of the Purinergic System in Neuromodulation.Brain Sci. 2024 Feb 16;14(2):180. doi: 10.3390/brainsci14020180. Brain Sci. 2024. PMID: 38391754 Free PMC article. Review.
-
Emerging Role of HDACs in Regeneration and Ageing in the Peripheral Nervous System: Repair Schwann Cells as Pivotal Targets.Int J Mol Sci. 2022 Mar 10;23(6):2996. doi: 10.3390/ijms23062996. Int J Mol Sci. 2022. PMID: 35328416 Free PMC article. Review.
-
EZH2-dependent myelination following sciatic nerve injury.Neural Regen Res. 2025 Aug 1;20(8):2382-2394. doi: 10.4103/NRR.NRR-D-23-02040. Epub 2024 May 13. Neural Regen Res. 2025. PMID: 39359095 Free PMC article.
-
The Role of Histone Deacetylases in NLRP3 Inflammasomesmediated Epilepsy.Curr Mol Med. 2024;24(8):980-1003. doi: 10.2174/1566524023666230731095431. Curr Mol Med. 2024. PMID: 37519210 Review.
-
Biological effects of sodium phenylbutyrate and taurursodiol in Alzheimer's disease.Alzheimers Dement (N Y). 2024 Aug 9;10(3):e12487. doi: 10.1002/trc2.12487. eCollection 2024 Jul-Sep. Alzheimers Dement (N Y). 2024. PMID: 39131742 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

