Acquired HIV drug resistance and virologic monitoring in a HIV hyper-endemic setting in KwaZulu-Natal Province, South Africa
- PMID: 34656129
- PMCID: PMC8520607
- DOI: 10.1186/s12981-021-00393-5
Acquired HIV drug resistance and virologic monitoring in a HIV hyper-endemic setting in KwaZulu-Natal Province, South Africa
Abstract
Background: Introduction of tenofovir (TDF) plus lamivudine (3TC) and dolutegravir (DTG) in first- and second-line HIV treatment regimens in South Africa warrants characterization of acquired HIV-1 drug resistance (ADR) mutations that could impact DTG-based antiretroviral therapy (ART). In this study, we sought to determine prevalence of ADR mutations and their potential impact on susceptibility to drugs used in combination with DTG among HIV-positive adults (≥ 18 years) accessing routine care at a selected ART facility in KwaZulu-Natal, South Africa.
Methods: We enrolled adult participants in a cross-sectional study between May and September 2019. Eligible participants had a most recent documented viral load (VL) ≥ 1000 copies/mL after at least 6 months on ART. We genotyped HIV-1 reverse transcriptase and protease genes by Sanger sequencing and assessed ADR. We characterized the effect of ADR mutations on the predicted susceptibility to drugs used in combination with DTG.
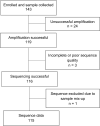
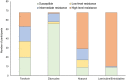
Results: From 143 participants enrolled, we obtained sequence data for 115 (80%), and 92.2% (95% CI 85.7-96.4) had ADR. The proportion with ADR was similar for participants on first-line ART (65/70, 92.9%, 95% CI 84.1-97.6) and those on second-line ART (40/44, 90.9%, 95% CI 78.3-97.5), and was present for the single participant on third-line ART. Approximately 89% (62/70) of those on first-line ART had dual class NRTI and NNRTI resistance and only six (13.6%) of those on second-line ART had major PI mutations. Most participants (82%) with first-line viraemia maintained susceptibility to Zidovudine (AZT), and the majority of them had lost susceptibility to TDF (71%) and 3TC (84%). Approximately two in every five TDF-treated individuals had thymidine analogue mutations (TAMs).
Conclusions: Susceptibility to AZT among most participants with first-line viraemia suggests that a new second-line regimen of AZT + 3TC + DTG could be effective. However, atypical occurrence of TAMs in TDF-treated individuals suggests a less effective AZT + 3TC + DTG regimen in a subpopulation of patients. As most patients with first-line viraemia had at least low-level resistance to TDF and 3TC, identifying viraemia before switch to TDF + 3TC + DTG is important to avoid DTG functional monotherapy. These findings highlight a need for close monitoring of outcomes on new standardized treatment regimens.
Keywords: Acquired drug resistance; Antiretroviral treatment; HIV-1; KwaZulu-Natal; Viraemia.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- UNAIDS. Fast-Track: ending the AIDS epidemic by 2030; 2014. https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014rep.... Accessed 25 Mar 2021.
-
- World Health Organization. progress report 2016: Prevent HIV, test and treat all: WHO support for country impact; 2016. https://apps.who.int/iris/handle/10665/251713. Accessed 05 Feb 2021.
-
- WHO. Policy brief: Update of recommendations on first-line antiretroviral regimens, July 2019; 2019. https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.1.... Accessed 25 Nov 2020.
-
- Mondi A, Cozzi-Lepri A, Tavelli A, Rusconi S, Vichi F, Ceccherini-Silberstein F, et al. Effectiveness of dolutegravir-based regimens as either first-line or switch antiretroviral therapy: data from the Icona cohort. J Int AIDS Soc. 2019;22:e25227–e25227. doi: 10.1002/jia2.25227. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

