Clinical outcomes and safety of polymyxin B in the treatment of carbapenem-resistant Gram-negative bacterial infections: a real-world multicenter study
- PMID: 34656132
- PMCID: PMC8520283
- DOI: 10.1186/s12967-021-03111-x
Clinical outcomes and safety of polymyxin B in the treatment of carbapenem-resistant Gram-negative bacterial infections: a real-world multicenter study
Abstract
Background: High morbidity and mortality due to carbapenem-resistant Gram-negative bacilli (CR-GNB) has led to the resurgence of polymyxin B (PMB) use in the last decade. The aim of our multicenter, real-world study was to evaluate the effectiveness and safety of PMB in the treatment of CR-GNB infections.
Methods: The real-world study included patients treated with intravenous PMB for at least 7 days during the period of October 2018 through June 2019. Associations between these clinical features and 28-day mortality or all-cause hospital mortality were explored through univariate analyses and multivariable logistic regression.
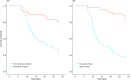
Results: The study included 100 patients. Many patients presented with combined chronic conditions, septic shock, mechanical ventilation, and the presence of Klebsiella pneumoniae. The mean duration of PMB therapy was 11 days (range 7-38 days). Temperature (38 °C vs 37.1 °C), white blood cells (14.13 × 109/l vs 9.28 × 109/l), C-reactive protein (103.55 ug/l vs 47.60 ug/l), procalcitonin (3.89 ng/ml vs 1.70 ng/ml) and APACHE II levels (17.75 ± 7.69 vs 15.98 ± 7.95) were significantly decreased after PMB treatment. The bacteria eradication rate was 77.65%. The overall mortality at discharge was 15%, and 28-day mortality was 40%. Major adverse reactions occurred in 16 patients. Nephrotoxicity was observed in 7 patients (7%).
Conclusions: Our results provide positive clinical and safety outcomes for PMB in the treatment of CR-GNB. Timely and appropriate use of PMB may be particularly useful in treating patients with sepsis in CR-GNB infections.
Keywords: 28-day mortality; Adverse effects; Carbapenem-resistant Gram-negative bacilli; Infections; Polymyxin B.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Early appropriate therapy with polymyxin B reduces the mortality in burn sepsis caused by carbapenem-resistant gram-negative bacteria: a retrospective analysis.Eur J Clin Microbiol Infect Dis. 2025 Jun;44(6):1433-1442. doi: 10.1007/s10096-025-05119-3. Epub 2025 Apr 3. Eur J Clin Microbiol Infect Dis. 2025. PMID: 40178717
-
Inhaled alone versus inhaled plus intravenous polymyxin B for the treatment of pneumonia due to carbapenem-resistant gram-negative bacteria: A prospective randomized controlled trial.Int J Antimicrob Agents. 2025 May;65(5):107483. doi: 10.1016/j.ijantimicag.2025.107483. Epub 2025 Feb 27. Int J Antimicrob Agents. 2025. PMID: 40023452 Clinical Trial.
-
Real-world data on the effects of colistin sulfate and polymyxin B sulfate in the treatment of pneumonia induced by CR-GNB.J Infect Dev Ctries. 2024 Jul 29;18(7):1050-1057. doi: 10.3855/jidc.18887. J Infect Dev Ctries. 2024. PMID: 39078788
-
The role of combination therapy in the treatment of severe infections caused by carbapenem resistant gram-negatives: a systematic review of clinical studies.BMC Infect Dis. 2021 Jun 9;21(1):545. doi: 10.1186/s12879-021-06253-x. BMC Infect Dis. 2021. PMID: 34107899 Free PMC article.
-
Systematic review and meta-analysis of in vitro efficacy of antibiotic combination therapy against carbapenem-resistant Gram-negative bacilli.Int J Antimicrob Agents. 2021 May;57(5):106344. doi: 10.1016/j.ijantimicag.2021.106344. Epub 2021 Apr 20. Int J Antimicrob Agents. 2021. PMID: 33857539
Cited by
-
Rapid Prediction of Multidrug-Resistant Klebsiella pneumoniae through Deep Learning Analysis of SERS Spectra.Microbiol Spectr. 2023 Mar 6;11(2):e0412622. doi: 10.1128/spectrum.04126-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36877048 Free PMC article.
-
The Efficacy and Influencing Factors of Polymyxin B in High-Level Carbapenem-Resistant Klebsiella pneumoniae Infections.Infect Drug Resist. 2023 Jun 27;16:4177-4187. doi: 10.2147/IDR.S409090. eCollection 2023. Infect Drug Resist. 2023. PMID: 37396067 Free PMC article.
-
Clinical Efficacy and Safety of Colistin Sulfate in the Treatment of Carbapenem-Resistant Organism Infections in Patients with Hematological Diseases.Infect Dis Ther. 2024 Jan;13(1):141-154. doi: 10.1007/s40121-023-00909-8. Epub 2024 Jan 11. Infect Dis Ther. 2024. PMID: 38212555 Free PMC article.
-
Chinese consensus guidelines for therapeutic drug monitoring of polymyxin B, endorsed by the Infection and Chemotherapy Committee of the Shanghai Medical Association and the Therapeutic Drug Monitoring Committee of the Chinese Pharmacological Society.J Zhejiang Univ Sci B. 2023 Feb 15;24(2):130-142. doi: 10.1631/jzus.B2200466. J Zhejiang Univ Sci B. 2023. PMID: 36751699 Free PMC article.
-
Polymyxin B-Based Regimens for Patients Infected with Carbapenem-Resistant Gram-Negative Bacteria: Clinical and Microbiological Efficacy, Mortality, and Safety.Infect Drug Resist. 2022 Mar 22;15:1205-1218. doi: 10.2147/IDR.S357746. eCollection 2022. Infect Drug Resist. 2022. PMID: 35345474 Free PMC article.
References
-
- Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400. doi: 10.1016/S1473-3099(18)30099-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U2004110/United Fund of National Natural Science Foundation of China
- 194200510017/Leading Talents Fund in Science and Technology Innovation in Henan Province
- 2019KJHM0001/Science and Technology people-benefit project of Zhengzhou
- Z-2018-35/The special fund for young and middle-aged medical research of China International Medical Exchange Foundation
- Z-2016-23-2001/The integrated thinking research foundation of the China foundation for International Medical Exchange
LinkOut - more resources
Full Text Sources
Research Materials

