A double-blind, placebo-controlled, randomized trial of PXT3003 for the treatment of Charcot-Marie-Tooth type 1A
- PMID: 34656144
- PMCID: PMC8520617
- DOI: 10.1186/s13023-021-02040-8
A double-blind, placebo-controlled, randomized trial of PXT3003 for the treatment of Charcot-Marie-Tooth type 1A
Erratum in
-
Correction to: A double-blind, placebo-controlled, randomized trial of PXT3003 for the treatment of Charcot-Marie-Tooth type 1A.Orphanet J Rare Dis. 2024 Apr 1;19(1):142. doi: 10.1186/s13023-024-03110-3. Orphanet J Rare Dis. 2024. PMID: 38561848 Free PMC article. No abstract available.
Abstract
Background: Charcot-Marie-Tooth disease type 1A (CMT1A) is a rare, orphan, hereditary neuromuscular disorder with no cure and for which only symptomatic treatment is currently available. A previous phase 2 trial has shown preliminary evidence of efficacy for PXT3003 in treating CMT1A. This phase 3, international, randomized, double-blind, placebo-controlled study further investigated the efficacy and safety of high- or low-dose PXT3003 (baclofen/naltrexone/D-sorbitol [mg]: 6/0.70/210 or 3/0.35/105) in treating subjects with mild to moderate CMT1A.
Methods: In this study, 323 subjects with mild-to-moderate CMT1A were randomly assigned in a 1:1:1 ratio to receive 5 mL of high- or low-dose PXT3003, or placebo, orally twice daily for up to 15 months. Efficacy was assessed using the change in Overall Neuropathy Limitations Scale total score from baseline to months 12 and 15 (primary endpoint). Secondary endpoints included the 10-m walk test and other assessments. The high-dose group was discontinued early due to unexpected crystal formation in the high-dose formulation, which resulted in an unanticipated high discontinuation rate, overall and especially in the high-dose group. The statistical analysis plan was adapted to account for the large amount of missing data before database lock, and a modified full analysis set was used in the main analyses. Two sensitivity analyses were performed to check the interpretation based on the use of the modified full analysis set.
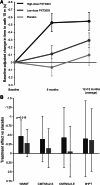
Results: High-dose PXT3003 demonstrated significant improvement in the Overall Neuropathy Limitations Scale total score vs placebo (mean difference: - 0.37 points; 97.5% CI [- 0.68 to - 0.06]; p = 0.008), and consistent treatment effects were shown in the sensitivity analyses. Both PXT3003 doses were safe and well-tolerated.
Conclusion: The high-dose group demonstrated a statistically significant improvement in the primary endpoint and a good safety profile. Overall, high-dose PXT3003 is a promising treatment option for patients with Charcot-Marie-Tooth disease type 1A.
Keywords: CMT1A; Charcot–Marie–Tooth; Neuromuscular disorder; Overall Neuropathy Limitations Scale; PMP22; PXT3003; Randomized controlled trial.
© 2021. The Author(s).
Conflict of interest statement
All authors were either paid as consultants by Pharnext or work at institutions that received funds from Pharnext to complete the study.
Figures


Similar articles
-
An exploratory randomised double-blind and placebo-controlled phase 2 study of a combination of baclofen, naltrexone and sorbitol (PXT3003) in patients with Charcot-Marie-Tooth disease type 1A.Orphanet J Rare Dis. 2014 Dec 18;9:199. doi: 10.1186/s13023-014-0199-0. Orphanet J Rare Dis. 2014. PMID: 25519680 Free PMC article. Clinical Trial.
-
Synergistic PXT3003 therapy uncouples neuromuscular function from dysmyelination in male Charcot-Marie-Tooth disease type 1A (CMT1A) rats.J Neurosci Res. 2020 Oct;98(10):1933-1952. doi: 10.1002/jnr.24679. Epub 2020 Jun 26. J Neurosci Res. 2020. PMID: 32588471
-
Early short-term PXT3003 combinational therapy delays disease onset in a transgenic rat model of Charcot-Marie-Tooth disease 1A (CMT1A).PLoS One. 2019 Jan 16;14(1):e0209752. doi: 10.1371/journal.pone.0209752. eCollection 2019. PLoS One. 2019. PMID: 30650121 Free PMC article.
-
Hereditary motor and sensory neuropathies or Charcot-Marie-Tooth diseases: an update.J Neurol Sci. 2014 Dec 15;347(1-2):14-22. doi: 10.1016/j.jns.2014.10.013. Epub 2014 Oct 16. J Neurol Sci. 2014. PMID: 25454638 Review.
-
Targeted Therapies for Hereditary Peripheral Neuropathies: Systematic Review and Steps Towards a 'treatabolome'.J Neuromuscul Dis. 2021;8(3):383-400. doi: 10.3233/JND-200546. J Neuromuscul Dis. 2021. PMID: 32773395 Free PMC article.
Cited by
-
Efficient data labeling strategies for automated muscle segmentation in lower leg MRIs of Charcot-Marie-Tooth disease patients.PLoS One. 2024 Sep 6;19(9):e0310203. doi: 10.1371/journal.pone.0310203. eCollection 2024. PLoS One. 2024. PMID: 39241036 Free PMC article.
-
Clinical trials in Charcot-Marie-Tooth disorders: a retrospective and preclinical assessment.Front Neurol. 2023 Sep 22;14:1251885. doi: 10.3389/fneur.2023.1251885. eCollection 2023. Front Neurol. 2023. PMID: 37808507 Free PMC article.
-
Correction to: A double-blind, placebo-controlled, randomized trial of PXT3003 for the treatment of Charcot-Marie-Tooth type 1A.Orphanet J Rare Dis. 2024 Apr 1;19(1):142. doi: 10.1186/s13023-024-03110-3. Orphanet J Rare Dis. 2024. PMID: 38561848 Free PMC article. No abstract available.
-
A Brief Review of Inherited Neuropathies: A Perspective from Saudi Arabia.Brain Sci. 2025 Apr 17;15(4):403. doi: 10.3390/brainsci15040403. Brain Sci. 2025. PMID: 40309874 Free PMC article. Review.
-
Charcot-Marie-Tooth disease: from historical landmarks in Brazil to current care perspectives.Arq Neuropsiquiatr. 2023 Oct;81(10):913-921. doi: 10.1055/s-0043-1770348. Epub 2023 Aug 23. Arq Neuropsiquiatr. 2023. PMID: 37611635 Free PMC article. Review.
References
-
- Abrams CK. GJB1 disorders: Charcot Marie Tooth neuropathy (CMT1X) and central nervous system phenotypes. In: Adam M, Ardinger H, Pagon R, et al., editors. GeneReviews. Seattle (WA); p. 1–23. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1374/#cmtx.Clinical_Characteristics. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

