Anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines: a systematic review and meta-analysis
- PMID: 34656181
- PMCID: PMC8520206
- DOI: 10.1186/s13223-021-00613-7
Anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines: a systematic review and meta-analysis
Abstract
Background: Currently there is no systematic review and meta-analysis of the global incidence rates of anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines in the general adult population.
Objectives: To estimate the incidence rates of anaphylactic and nonanaphylactic reactions after COVID-19 vaccines and describe the demographic and clinical characteristics, triggers, presenting signs and symptoms, treatment and clinical course of confirmed cases.
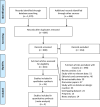
Design: A systematic review and meta-analysis. Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] statement was followed.
Methods: Electronic databases (Proquest, Medline, Embase, Pubmed, CINAHL, Wiley online library, and Nature) were searched from 1 December 2020 to 31 May 2021 in the English language using the following keywords alone or in combination: anaphylaxis, non-anaphylaxis, anaphylactic reaction, nonanaphylactic reaction, anaphylactic/anaphylactoid shock, hypersensitivity, allergy reaction, allergic reaction, immunology reaction, immunologic reaction, angioedema, loss of consciousness, generalized erythema, urticaria, urticarial rash, cyanosis, grunting, stridor, tachypnoea, wheezing, tachycardia, abdominal pain, diarrhea, nausea, vomiting and tryptase. We included studies in adults of all ages in all healthcare settings. Effect sizes of prevalence were pooled with 95% confidence intervals (CIs). To minimize heterogeneity, we performed sub-group analyses.
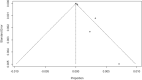
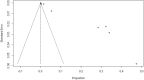
Results: Of the 1,734 papers that were identified, 26 articles were included in the systematic review (8 case report, 5 cohort, 4 case series, 2 randomized controlled trial and 1 randomized cross-sectional studies) and 14 articles (1 cohort, 2 case series, 1 randomized controlled trial and 1 randomized cross-sectional studies) were included in meta-analysis. Studies involving 26,337,421 vaccine recipients [Pfizer-BioNTech (n = 14,505,399) and Moderna (n = 11,831,488)] were analyzed. The overall pooled prevalence estimate of anaphylaxis to both vaccines was 5.0 (95% CI 2.9 to 7.2, I2 = 81%, p = < 0.0001), while the overall pooled prevalence estimate of nonanaphylactic reactions to both vaccines was 53.9 (95% CI 0.0 to 116.1, I2 = 99%, p = < 0.0001). Vaccination with Pfizer-BioNTech resulted in higher anaphylactic reactions compared to Moderna (8.0, 95% CI 0.0 to 11.3, I2 = 85% versus 2.8, 95% CI 0.0 to 5.7, I2 = 59%). However, lower incidence of nonanaphylactic reactions was associated with Pfizer-BioNTech compared to Moderna (43.9, 95% CI 0.0 to 131.9, I2 = 99% versus 63.8, 95% CI 0.0 to 151.8, I2 = 98%). The funnel plots for possible publication bias for the pooled effect sizes to determine the incidence of anaphylaxis and nonanaphylactic reactions associated with mRNA COVID-19 immunization based on mRNA vaccine type appeared asymmetrical on visual inspection, and Egger's tests confirmed asymmetry by producing p values < 0.05. Across the included studies, the most commonly identified risk factors for anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines were female sex and personal history of atopy. The key triggers to anaphylactic and nonanaphylactic reactions identified in these studies included foods, medications, stinging insects or jellyfish, contrast media, cosmetics and detergents, household products, and latex. Previous history of anaphylaxis; and comorbidities such as asthma, allergic rhinitis, atopic and contact eczema/dermatitis and psoriasis and cholinergic urticaria were also found to be important.
Conclusion: The prevalence of COVID-19 mRNA vaccine-associated anaphylaxis is very low; and nonanaphylactic reactions occur at higher rate, however, cutaneous reactions are largely self-limited. Both anaphylactic and nonanaphylactic reactions should not discourage vaccination.
Keywords: Allergic; COVID-19; Immunologic; Incidence; Reactions; SARS-Cov-2; Systematic review; Vaccines.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard 2021. https://covid19.who.int. . Accessed 28 May 2021.
-
- United States Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise.... Accessed 6 Jun 2021.
-
- United States Food and Drug Administration. Moderna COVID-19 Vaccine 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise.... Accessed 6 Jun 2021.
-
- Temsah M-H, Barry M, Aljamaan F, Alhuzaimi AN, Al-Eyadhy A, Saddik B, Alsohime F, Alhaboob A, Alhasan K, Alaraj A. SARS-CoV-2 B. 1.1. 7 UK variant of concern lineage-related perceptions, COVID-19 vaccine acceptance and travel worry among healthcare workers. Front Public Health. 2021;9:617. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

