Mitochondrial iron-sulfur clusters: Structure, function, and an emerging role in vascular biology
- PMID: 34656823
- PMCID: PMC8577454
- DOI: 10.1016/j.redox.2021.102164
Mitochondrial iron-sulfur clusters: Structure, function, and an emerging role in vascular biology
Abstract
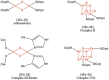
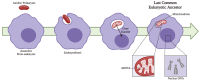
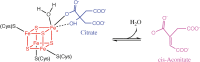
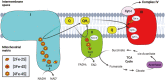
Iron-sulfur (Fe-S) clusters are essential cofactors most commonly known for their role mediating electron transfer within the mitochondrial respiratory chain. The Fe-S cluster pathways that function within the respiratory complexes are highly conserved between bacteria and the mitochondria of eukaryotic cells. Within the electron transport chain, Fe-S clusters play a critical role in transporting electrons through Complexes I, II and III to cytochrome c, before subsequent transfer to molecular oxygen. Fe-S clusters are also among the binding sites of classical mitochondrial inhibitors, such as rotenone, and play an important role in the production of mitochondrial reactive oxygen species (ROS). Mitochondrial Fe-S clusters also play a critical role in the pathogenesis of disease. High levels of ROS produced at these sites can cause cell injury or death, however, when produced at low levels can serve as signaling molecules. For example, Ndufs2, a Complex I subunit containing an Fe-S center, N2, has recently been identified as a redox-sensitive oxygen sensor, mediating homeostatic oxygen-sensing in the pulmonary vasculature and carotid body. Fe-S clusters are emerging as transcriptionally-regulated mediators in disease and play a crucial role in normal physiology, offering potential new therapeutic targets for diseases including malaria, diabetes, and cancer.
Keywords: Drug target; Electron transport chain; Epigenetics; Fe-S cluster; Mitochondria; Oxygen-sensing.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Ewen K.M., Ringle M., Bernhardt R. Adrenodoxin–a versatile ferredoxin. IUBMB Life. 2012;64(6):506–512. - PubMed
-
- Ali M.E., Nair N.N., Retegan M., Neese F., Staemmler V., Marx D. The iron–sulfur core in Rieske proteins is not symmetric. JBIC Journal of Biological Inorganic Chemistry. 2014;19(8):1287–1293. - PubMed
-
- Ohnishi T. Iron–sulfur clusters/semiquinones in Complex I, Biochimica et Biophysica Acta (BBA) Bioenergetics. 1998;1364(2):186–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

