Lactate in the tumour microenvironment: From immune modulation to therapy
- PMID: 34656878
- PMCID: PMC8524104
- DOI: 10.1016/j.ebiom.2021.103627
Lactate in the tumour microenvironment: From immune modulation to therapy
Abstract
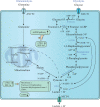
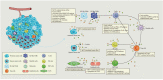
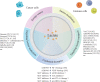
Disordered metabolic states, which are characterised by hypoxia and elevated levels of metabolites, particularly lactate, contribute to the immunosuppression in the tumour microenvironment (TME). Excessive lactate secreted by metabolism-reprogrammed cancer cells regulates immune responses via causing extracellular acidification, acting as an energy source by shuttling between different cell populations, and inhibiting the mechanistic (previously 'mammalian') target of rapamycin (mTOR) pathway in immune cells. This review focuses on recent advances in the regulation of immune responses by lactate, as well as therapeutic strategies targeting lactate anabolism and transport in the TME, such as those involving glycolytic enzymes and monocarboxylate transporter inhibitors. Considering the multifaceted roles of lactate in cancer metabolism, a comprehensive understanding of how lactate and lactate-targeting therapies regulate immune responses in the TME will provide insights into the complex relationships between metabolism and antitumour immunity.
Keywords: Cancer immunity; Cancer metabolism; Glycolytic enzymes; Lactate; Monocarboxylate transporters; Tumour microenvironment.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest All authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

