Effect of febuxostat on left ventricular diastolic function in patients with asymptomatic hyperuricemia: a sub analysis of the PRIZE Study
- PMID: 34657137
- PMCID: PMC8668434
- DOI: 10.1038/s41440-021-00752-9
Effect of febuxostat on left ventricular diastolic function in patients with asymptomatic hyperuricemia: a sub analysis of the PRIZE Study
Abstract
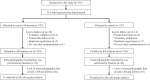
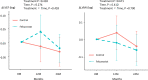
Hyperuricemia is related to an increased risk of cardiovascular events from a meta-analysis and antihyperuricemia agents may influence to cardiac function. We evaluated the effect of febuxostat on echocardiographic parameters of diastolic function in patients with asymptomatic hyperuricemia as a prespecified endpoint in the subanalysis of the PRIZE study. Patients in the PRIZE study were assigned randomly to either add-on febuxostat treatment group or control group with only appropriate lifestyle modification. Of the 514 patients in the overall study, 65 patients (31 in the febuxostat group and 34 in the control group) who had complete follow-up echocardiographic data of the ratio of peak early diastolic transmitral flow velocity (E) to peak early diastolic mitral annular velocity (e') at baseline and after 12 and 24 months were included. The primary endpoint was a comparison of the changes in the E/e' between the two groups from baseline to 24 months. Interestingly, e' was slightly decreased in the control group compared with in the febuxostat group (treatment p = 0.068, time, p = 0.337, treatment × Time, p = 0.217). As a result, there were significant increases in E/e' (treatment p = 0.045, time, p = 0.177, treatment × time, p = 0.137) after 24 months in the control group compared with the febuxostat group. There was no significant difference in the serum levels of N-terminal-pro brain natriuretic peptide and high-sensitive troponin I between the two groups during the study period. In conclusions, additional febuxostat treatment in patients with asymptomatic hyperuricemia for 24 months might have a potential of preventable effects on the impaired diastolic dysfunction.
Keywords: NT-proBNP; diastolic function; echocardiography; febuxostat; hyperuricemia.
© 2021. The Author(s).
Conflict of interest statement
A.T. has received honoraria from Boehringer Ingelheim and research funding from GlaxoSmithKline. H.T. received honoraria from Abbott Medical Japan, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Kowa, Ono, Mitsubishi Tanabe and Takeda. Y.F. received research grant from Sanofi KK and Shionogi & Co. Ltd., honoraria from Public Health Research Foundation, AstraZeneca KK, Eisai Co. Ltd., Kowa Pharmaceutical Co. Ltd., and Ono Pharmaceutical Co. Ltd., and research grant and honoraria from MSD KK, Otsuka Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., Teijin Pharma Ltd., Bayer Yakuhin, Ltd., Mochida Pharmaceutical Co. Ltd., Astellas Pharma Inc., Sanwa Kagaku Kenkyusho Co. Ltd., Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corp., and Pfizer Japan Inc. K. Kario. has received research grant, honoraria and consulting fees from Sanwa Kagaku Kenkyusho Co. M.S. has received honoraria from Bayer, Boehringer Ingelheim, Takeda, Astellas, Mochida, Mitsubishi Tanabe, Pfizer, Novartis; research funding from Boehringer Ingelheim; scholarships from Astellas, Takeda, Daiichi Sankyo, MSD, Novartis, Boehringer Ingelheim, AstraZeneca, and Pfizer. K.N. has received research grants from Asahi Kasei, Astellas, Bayer, Boehringer Ingelheim, Mitsubishi Tanabe, Teijin, and Terumo; scholarships from Astellas, Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Daiichi Sankyo Healthcare, Takeda, and Teijin; and personal fees from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo Healthcare, Eli Lilly, Kowa, Mitsubishi Tanabe, MSD, Novartis, Ono, Takeda, and Teijin. All other authors declare no competing interests.
Figures



Comment in
-
The dawn of a new era of targeted therapies for heart failure with preserved ejection fraction (HFpEF).Hypertens Res. 2022 Jan;45(1):164-166. doi: 10.1038/s41440-021-00799-8. Epub 2021 Nov 24. Hypertens Res. 2022. PMID: 34819620 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

