A positive feedback loop of lncRNA-RMRP/ZNRF3 axis and Wnt/β-catenin signaling regulates the progression and temozolomide resistance in glioma
- PMID: 34657141
- PMCID: PMC8520527
- DOI: 10.1038/s41419-021-04245-y
A positive feedback loop of lncRNA-RMRP/ZNRF3 axis and Wnt/β-catenin signaling regulates the progression and temozolomide resistance in glioma
Abstract
Drug resistance strikingly limits the therapeutic effect of temozolomide (TMZ) (a common drug for glioma). Long non-coding RNA (lncRNA) RMRP has been found to be implicated in glioma progression. However, the effect of RMRP on TMZ resistance along with related molecular mechanisms is poorly defined in glioma. In the present study, RMRP, ZNRF3, and IGF2BP3 were screened out by bioinformatics analysis. The expression levels of lncRNAs and mRNAs were measured by RT-qPCR assay. Protein levels of genes were detected by western blot and immunofluorescence assays. ZNRF3 mRNA stability was analyzed using Actinomycin D assay. Cell proliferative ability and survival rate were determined by CCK-8 assay. Cell apoptotic pattern was estimated by flow cytometry. The effect of RMRP knockdown on the growth of TMZ-treated glioma xenograft tumors was explored in vivo. The relationships of IGF2BP3, RMRP, and ZNRF3 were explored by bioinformatics prediction analysis, RNA immunoprecipitation, luciferase, and RNA pull-down, and chromatin immunoprecipitation assays. The results showed that RMRP was highly expressed in glioma. RMRP knockdown curbed cell proliferation, facilitated cell apoptosis and reduced TMZ resistance in glioma cells, and hindered the growth of TMZ-treated glioma xenograft tumors. RMRP exerted its functions by down-regulating ZNRF3 in glioma cells. IGF2BP3 interacted with RMRP and ZNRF3 mRNA. IGF2BP3 knockdown weakened the interaction of Argonaute 2 (Ago2) and ZNRF3. RMRP reduced ZNRF3 expression and mRNA stability by IGF2BP3. RMRP knockdown inhibited β-catenin expression by up-regulating ZNRF3. The inhibition of Wnt/β-catenin signaling pathway by XAV-939 weakened RMRP-mediated TMZ resistance in glioma cells. β-catenin promoted RMRP expression by TCF4 in glioma cells. In conclusion, RMRP/ZNRF3 axis and Wnt/β-catenin signaling formed a positive feedback loop to regulate TMZ resistance in glioma. The sustained activation of Wnt/β-catenin signaling by RMRP might contribute to the better management of cancers.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


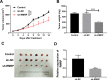




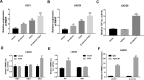
Similar articles
-
MicroRNA‑301a/ZNRF3/wnt/β‑catenin signal regulatory crosstalk mediates glioma progression.Int J Oncol. 2021 Jan;58(1):45-56. doi: 10.3892/ijo.2020.5145. Epub 2020 Nov 10. Int J Oncol. 2021. PMID: 33367931 Free PMC article.
-
MSC-AS1 knockdown inhibits cell growth and temozolomide resistance by regulating miR-373-3p/CPEB4 axis in glioma through PI3K/Akt pathway.Mol Cell Biochem. 2021 Feb;476(2):699-713. doi: 10.1007/s11010-020-03937-x. Epub 2020 Oct 26. Mol Cell Biochem. 2021. PMID: 33106913 Free PMC article.
-
LINC00511 facilitates Temozolomide resistance of glioblastoma cells via sponging miR-126-5p and activating Wnt/β-catenin signaling.J Biochem Mol Toxicol. 2021 Sep;35(9):e22848. doi: 10.1002/jbt.22848. Epub 2021 Jul 30. J Biochem Mol Toxicol. 2021. PMID: 34328678
-
Function of Long Noncoding RNAs in Glioma Progression and Treatment Based on the Wnt/β-Catenin and PI3K/AKT Signaling Pathways.Cell Mol Neurobiol. 2023 Nov;43(8):3929-3942. doi: 10.1007/s10571-023-01414-9. Epub 2023 Sep 25. Cell Mol Neurobiol. 2023. PMID: 37747595 Free PMC article. Review.
-
Opposite Interplay Between the Canonical WNT/β-Catenin Pathway and PPAR Gamma: A Potential Therapeutic Target in Gliomas.Neurosci Bull. 2018 Jun;34(3):573-588. doi: 10.1007/s12264-018-0219-5. Epub 2018 Mar 26. Neurosci Bull. 2018. PMID: 29582250 Free PMC article. Review.
Cited by
-
Targeting IGF2BP3 in Cancer.Int J Mol Sci. 2023 May 29;24(11):9423. doi: 10.3390/ijms24119423. Int J Mol Sci. 2023. PMID: 37298373 Free PMC article. Review.
-
Predicting Treatment Outcomes in Glioblastoma: A Risk Score Model for TMZ Resistance and Immune Checkpoint Inhibition.Biology (Basel). 2025 May 20;14(5):572. doi: 10.3390/biology14050572. Biology (Basel). 2025. PMID: 40427760 Free PMC article.
-
Long Non-Coding RNAs in Malignant Human Brain Tumors: Driving Forces Behind Progression and Therapy.Int J Mol Sci. 2025 Jan 15;26(2):694. doi: 10.3390/ijms26020694. Int J Mol Sci. 2025. PMID: 39859408 Free PMC article. Review.
-
Non-coding RNA-Mediated N6-Methyladenosine (m6A) deposition: A pivotal regulator of cancer, impacting key signaling pathways in carcinogenesis and therapy response.Noncoding RNA Res. 2023 Nov 13;9(1):84-104. doi: 10.1016/j.ncrna.2023.11.005. eCollection 2024 Mar. Noncoding RNA Res. 2023. PMID: 38075202 Free PMC article. Review.
-
Shedding light on function of long non-coding RNAs (lncRNAs) in glioblastoma.Noncoding RNA Res. 2024 Feb 6;9(2):508-522. doi: 10.1016/j.ncrna.2024.02.002. eCollection 2024 Jun. Noncoding RNA Res. 2024. PMID: 38511060 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

