Tyrosine phosphatases regulate resistance to ALK inhibitors in ALK+ anaplastic large cell lymphoma
- PMID: 34657149
- PMCID: PMC8814675
- DOI: 10.1182/blood.2020008136
Tyrosine phosphatases regulate resistance to ALK inhibitors in ALK+ anaplastic large cell lymphoma
Abstract
Anaplastic large cell lymphomas (ALCLs) frequently carry oncogenic fusions involving the anaplastic lymphoma kinase (ALK) gene. Targeting ALK using tyrosine kinase inhibitors (TKIs) is a therapeutic option in cases relapsed after chemotherapy, but TKI resistance may develop. By applying genomic loss-of-function screens, we identified PTPN1 and PTPN2 phosphatases as consistent top hits driving resistance to ALK TKIs in ALK+ ALCL. Loss of either PTPN1 or PTPN2 induced resistance to ALK TKIs in vitro and in vivo. Mechanistically, we demonstrated that PTPN1 and PTPN2 are phosphatases that bind to and regulate ALK phosphorylation and activity. In turn, oncogenic ALK and STAT3 repress PTPN1 transcription. We found that PTPN1 is also a phosphatase for SHP2, a key mediator of oncogenic ALK signaling. Downstream signaling analysis showed that deletion of PTPN1 or PTPN2 induces resistance to crizotinib by hyperactivating SHP2, the MAPK, and JAK/STAT pathways. RNA sequencing of patient samples that developed resistance to ALK TKIs showed downregulation of PTPN1 and PTPN2 associated with upregulation of SHP2 expression. Combination of crizotinib with a SHP2 inhibitor synergistically inhibited the growth of wild-type or PTPN1/PTPN2 knock-out ALCL, where it reverted TKI resistance. Thus, we identified PTPN1 and PTPN2 as ALK phosphatases that control sensitivity to ALK TKIs in ALCL and demonstrated that a combined blockade of SHP2 potentiates the efficacy of ALK inhibition in TKI-sensitive and -resistant ALK+ ALCL.
© 2022 by The American Society of Hematology.
Figures
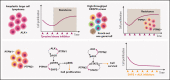




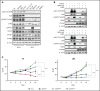




Comment in
-
Getting ALK inhibitors SHPshape.Blood. 2022 Feb 3;139(5):642-643. doi: 10.1182/blood.2021014301. Blood. 2022. PMID: 35113153 No abstract available.
Similar articles
-
Targeting CCR7-PI3Kγ overcomes resistance to tyrosine kinase inhibitors in ALK-rearranged lymphoma.Sci Transl Med. 2023 Jun 28;15(702):eabo3826. doi: 10.1126/scitranslmed.abo3826. Epub 2023 Jun 28. Sci Transl Med. 2023. PMID: 37379367 Free PMC article.
-
PTPN2 Inhibition Disrupts Mitochondrial Renewal and Blocks TFRC-Mediated Mitophagy to Exert Anti-Tumor Activities in ALK-Positive Anaplastic Large Cell Lymphoma.Adv Sci (Weinh). 2025 Aug;12(31):e14282. doi: 10.1002/advs.202414282. Epub 2025 Jul 30. Adv Sci (Weinh). 2025. PMID: 40734623 Free PMC article.
-
Blockade of crizotinib-induced BCL2 elevation in ALK-positive anaplastic large cell lymphoma triggers autophagy associated with cell death.Haematologica. 2019 Jul;104(7):1428-1439. doi: 10.3324/haematol.2017.181966. Epub 2019 Jan 24. Haematologica. 2019. PMID: 30679328 Free PMC article.
-
Nucleophosmin-anaplastic lymphoma kinase: the ultimate oncogene and therapeutic target.Blood. 2017 Feb 16;129(7):823-831. doi: 10.1182/blood-2016-05-717793. Epub 2016 Nov 22. Blood. 2017. PMID: 27879258 Review.
-
Second Paediatric Strategy Forum for anaplastic lymphoma kinase (ALK) inhibition in paediatric malignancies: ACCELERATE in collaboration with the European Medicines Agency with the participation of the Food and Drug Administration.Eur J Cancer. 2021 Nov;157:198-213. doi: 10.1016/j.ejca.2021.08.022. Epub 2021 Sep 15. Eur J Cancer. 2021. PMID: 34536944 Review.
Cited by
-
Obesity and Risk for Lymphoma: Possible Role of Leptin.Int J Mol Sci. 2022 Dec 8;23(24):15530. doi: 10.3390/ijms232415530. Int J Mol Sci. 2022. PMID: 36555171 Free PMC article. Review.
-
Targeting ERBB3 and AKT to overcome adaptive resistance in EML4-ALK-driven non-small cell lung cancer.Cell Death Dis. 2024 Dec 18;15(12):912. doi: 10.1038/s41419-024-07272-7. Cell Death Dis. 2024. PMID: 39695132 Free PMC article.
-
Tyrosine kinases in nodal peripheral T-cell lymphomas.Front Oncol. 2023 Feb 8;13:1099943. doi: 10.3389/fonc.2023.1099943. eCollection 2023. Front Oncol. 2023. PMID: 36845713 Free PMC article. Review.
-
Case report: Musculoskeletal metastastic inflammatory myofibroblastic tumor (IMT) treated by sequential ALK-TKI with longterm response.Front Oncol. 2025 Jan 27;14:1505257. doi: 10.3389/fonc.2024.1505257. eCollection 2024. Front Oncol. 2025. PMID: 39931211 Free PMC article.
-
Protein Tyrosine Phosphatases in Neuroblastoma: Emerging Roles as Biomarkers and Therapeutic Targets.Front Cell Dev Biol. 2021 Dec 8;9:811297. doi: 10.3389/fcell.2021.811297. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34957126 Free PMC article. Review.
References
-
- Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1):11-23. - PubMed
-
- Gambacorti Passerini C, Farina F, Stasia A, et al. . Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J Natl Cancer Inst. 2014;106(2):djt378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

